THIS ARTICLE/PRESS RELEASE IS PAID FOR AND PRESENTED BY University of Oslo - read more

Chemists used Poirot’s logic to investigate the Grignard reaction
What is the similarity between "Murder on the Orient Express" and the Grignard reaction? “All the suspects were guilty”, say two researchers.
The two researchers at the University of Oslo wondered whether their new scientific paper was going to attract some attention when it was published, because they had managed to unravel the innermost secrets of the old Grignard reaction. This famous and important chemical reaction had remained poorly understood since the French chemist Victor Grignard discovered it some 120 years ago, but “Good old Grignard” gave it all up when Eisenstein and Cascella applied modern technology in their investigation.
When the paper was finally published, it gained much larger attention than Eisenstein and Cascella had expected. In fact, they spent the next few days just looking up the numbers, counting and rejoicing. “Look here, we have had 1000 views on our article! No, now it's 2000 views! And this continued for quite some time,” Professor Michele Cascella remembers.
The scientific article was published in mid-January 2020 in the Journal of the American Chemical Society. Nine months later, the article had been read no less than 24,000 times. This is what counts as a bestseller when it comes to scientific articles about organic chemistry.
The attention is partly due to the fact that Grignard is one of the world's most important and useful chemical reactions, present in the programs of basic courses in organic chemistry at universities all over the world.
But nobody knew how the reaction really worked when Eisenstein and Cascella began their detective work five or six years ago. In other words: the Grignard reaction had long been seen as a "cold case"; that is, as an old and unsolved mystery which can only be solved by the best detectives with the most advanced techniques.
The birth of organic chemistry
“Actually, the French chemist Victor Grignard discovered much more than the reaction that was named after him,” says Cascella.
“The discovery also served as the birth of modern organic chemistry. This was the first reaction that could be used to tailor chemical bonds between carbon atoms,” he explains.
Professor Odile Eisenstein agrees:

“When you have some specific molecules and want to use them to produce other molecules, you actually have to break chemical bonds between some atoms and establish new chemical bonds between other atoms. But it is difficult to break strong bonds, and the bonds between carbon atoms are very strong. Victor Grignard gave us the first reaction that could be used to break and form new bonds between carbon atoms.”
The importance of Victor Grignard's discovery was immediately recognized by other researchers, to the point that he received the Nobel Prize in Chemistry only 12 years later after. Since then, the reaction has been used to make such a large number of useful industrial products that it is impossible to list them all.
“I find it extra impressive that Grignard discovered this reaction before the nature of chemical bonding was discovered at all, and even before the structure of the atom was properly understood. Grignard also made his discovery only three years after Joseph John Thomson’s discovery of the electron,” Cascella explains.
The beginning of the 20th century was a time of great discoveries in chemistry. Grignard's discovery occurred in the same year that Max Planck defined the constant that bears his name, and which has later become a key player in quantum mechanics. A few years after Grignard’s discovery, Ernest Rutherford discovered the atomic nucleus and was able to describe the structure of the atom; that happened in 1911.

This is the Grignard reaction
Victor Grignard first discovered that finely crushed magnesium can be dissolved in ether mixed with bromoalkene. This leads to the formation of a substance that has later been called the Grignard reagent, which again can react with many different substances such as ketones, aldehydes and alkenes. An alkene, by the way, is a chemical substance that consists only of carbon and hydrogen and is "unsaturated" – in the sense that at least two carbon atoms are attached to each other by a double bond. When the substance is called bromoalkene, the name means that at least one of the hydrogen atoms has been replaced with bromine.
The Grignard reaction leads to the formation of alcohols based on the original molecules, and the alcohols can again be used as raw material in the production of a large number of new products. This reaction is not only very useful, it is also easy to run. You can basically start the whole process just by shaking the ingredients in a test tube.
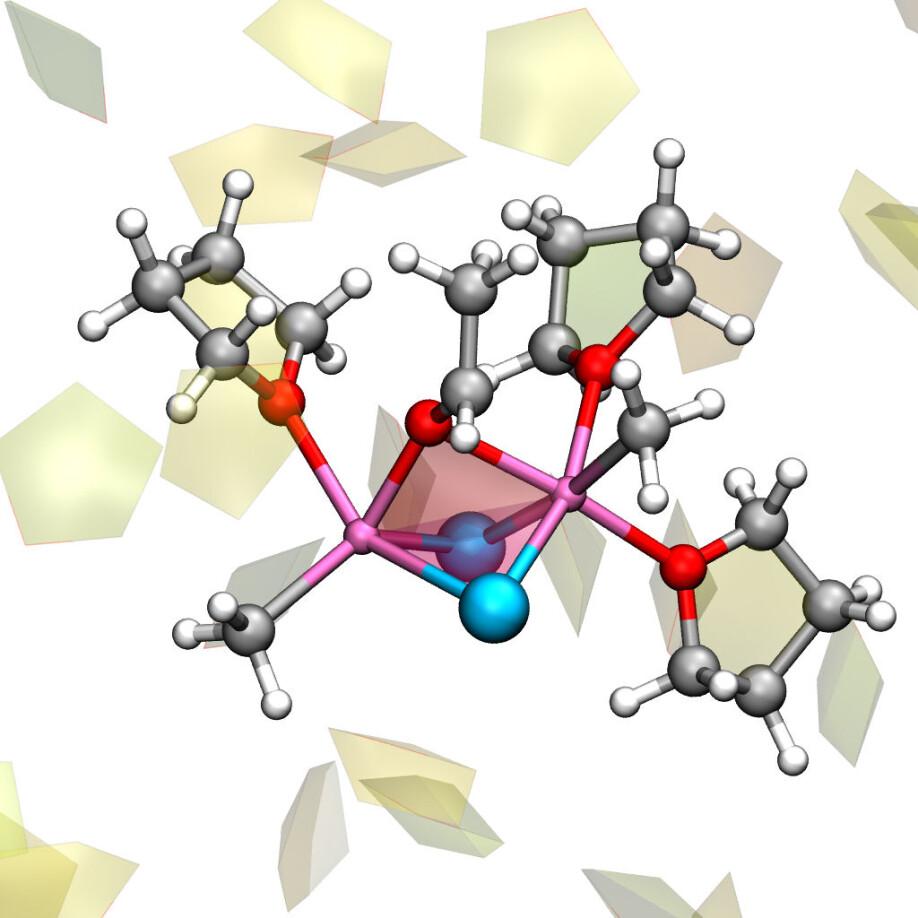
But – what did this Grignard reagent look like? The chemists at the beginning of the 20th century were at a loss, but they understood that they were looking at a negatively charged organic compound with magnesium. The most important thing was anyway that the reaction worked, and that it did what chemists wanted it to do.
“But it has been very frustrating for us chemists that we did not understand how such a basic chemical reaction works at the molecular level. This lack of knowledge led to, among other things, that it became very difficult to optimize the process,” Eisenstein comments. But now that the reaction is understood, researchers can start to refine it, replacing a molecule here and adjusting the temperature there, and in general doing the things that chemists are experts at.
All usual suspects were guilty
To make a long story short: Eisenstein and Cascella have shown that the Grignard reagent is not a single molecule, but instead a variable molecule that can change both its form and the chemical composition. They have managed to map an entire process in which the molecules in the solvent attach to the magnesium atoms and leave them again, in a kind of molecular dance.
This means that there are always many slightly different Grignard reagents in the chemical reaction, interchanging through what the researchers call a Schlenk equilibrium – named after its discoverer Wilhelm Schlenk. But which variants of the Grignard reagent actually reacted and led to the formation of useful substances?
This was the great mystery; let us skip right to the conclusion:
“You have probably read Agatha Christie's crime novel Murder on the Orient Express or seen one of the film adaptations. In this murder mystery, the master detective Hercule Poirot finally discovers that all those on the train are guilty, and this is also the case in the Grignard reaction. All suspects contribute in their own way! You could say that one reagent opens the door while the other keeps an eye out for the police and the third overpowers the murder victim, and so on,” Eisenstein says.
“Some of the Grignard reagents work very fast, others are a little slower, but all of them contribute to the process. This is why the chemists who studied reactions in the laboratories were unable to describe it. The Grignard reaction is actually a whole group of chemical reactions that take place simultaneously in the same solution,” Cascella explains.
Calculations instead of experiments in the lab
Michele Cascella and Odile Eisenstein have not used traditional and recognized laboratory methods such as mass spectrometry and spectroscopy to unravel the innermost secrets of the Grignard reaction. Instead, they have used mathematical modelling and computer simulation, which is a main activity at the Hylleraas Centre where both are employed as researchers.
The Hylleraas Centre is a Centre of Excellence at the University of Oslo and the Arctic University of Norway - Tromsø. At the centre, some 50-60 researchers are going to use the years until 2027 to investigate complex chemical systems, virtually wherever they may be found.
Their goal is what the centre’s leaders call the "Holy Grail of Chemistry": They are studying how mathematical modelling and computer simulations can be used to calculate how new chemical bonds can be broken and made in a complex environment – and this opens the door for the production of completely new, tailor-made chemical substances.
In other words, the Hylleraas Centre was just the right place for two researchers who wanted to reveal the secrets of the Grignard reaction. Here, they had the opportunity to simulate and model both the reagents and the solvents in the reaction in a realistic way.
Odile Eisenstein talks about "dancing pairs" when she talks about the many reactions that goes on inside the Schlenk equilibrium. The dancing starts for example when one molecule reacts with another molecule in a way that leads to the exchange of a magnesium molecule between them.
“First of all, we found out that almost all dance pairs ended up forming new and stable bonds between carbon atoms. Secondly: We found that the different dance couples react via different mechanisms. You might say that every couple has its own moves in the dance,” Eisenstein says.
Started for fun in 2014
Odile Eisenstein and Michele Cascella have worked on an off with the Grignard reaction between other things for several years, but the collaboration between them really began in September 2014. Cascella had recently been employed as an associate professor of theoretical chemistry at the University of Oslo, and Eisenstein was already an internationally respected researcher in the subject of computational chemistry.
When Eisenstein came to Oslo in order to receive an honorary doctorate at UiO, Cascella naturally went to witness the ceremony. During the award ceremony, Eisenstein gave an enthusiastic speech about all the wonderful things that were becoming possible with the help of computational chemistry. Professor Mats Tilset invited her to say a few words about which chemical reactions were still poorly understood, and Eisenstein responded by claiming that the Grignard reaction was the prototype of such a reaction. Maybe it was even too complex to be understood, Eisenstein said.
That statement caught Cascella’s interest at once, because “Good old Grignard” was the first organic chemical reaction he had learned about as a young student at La Sapienza University in Rome 25 years ago.
“I did not know that this reaction was so poorly understood, so I contacted Odile afterwards because I thought it should be possible to solve this problem. Thus, we both began to look more closely at the Grignard reaction, at first mostly for fun. But later on, the project became more and more interesting,” says Cascella.
Knowledge creates improvement
Now that the researchers have figured out how the Grignard reaction really goes, people can start the painstaking work of improving it. Researchers in chemistry are constantly working to refine familiar processes by such things as replacing reagents that are toxic and/or expensive with other reagents that are inexpensive and harmless.
In this case, magnesium is neither particularly expensive nor toxic. But perhaps it is possible to come up with a clever trick that makes the Grignard reaction work with other metals – maybe even sodium? Sodium is one of the cheapest and non-dangerous elements available, and everybody has a sodium compound in their kitchen and use it all the time.
“Pure sodium alone is simply too reactive, it would just "burn up" the other ingredients in Grignard. But maybe we can find additives that make it possible to use sodium anyway. Regardless, we hope that the new understanding of the Grignard reaction can contribute to the development of a new, green, renewable and inexpensive chemistry based on non-toxic elements,” Cascella dreams.
“In principle, it is always better with more knowledge that enables us to understand the basic aspects of what we work with. When we know how to bake a cake, we can also find out how we can use new spices and additives to bake a cake that tastes even better and looks more beautiful. There is still a way to go before new applications can grow out of our work on Grignard, but we really believe that we have taken an important step forward. Now, we know in which direction we should go,” comments Eisenstein.
Odile Eisenstein and Michele Cascella have had important collaborators in their work. Professor Jürgen Gauss at the Johannes Gutenberg University in Mainz (Germany) is an expert in quantum chemistry and made a significant contribution to identifying the sequence of the various reactions that occur in the process. The first author of the article is Raphael Mathias Peltzer, who studied the Grignard reaction as a part of his doctorate thesis at UiO in 2018.
References:
Raphael Mathias Peltzer et.al.: The Grignard Reaction – Unraveling a Chemical Puzzle. Journal of the American Chemical Society, 2020.
Raphael Mathias Peltzer et.al.: How Solvent Dynamics Controls the Schlenk Equilibrium of Grignard Reagents: A Computational Study of CH3MgCl in Tetrahydrofuran. Journal of Physical Chemistry B, 2017.
David Balcells et.al.: A Career in Catalysis: Odile Eisenstein. ACS Catalysis, 2019. Summary.
Dietmar Seyferth: The Grignard Reagents. Organometallics, 2009.
Georges Bram et.al.: Victor Grignard et la naissance de son réactif. Comptes Rendus de l'Académie des Sciences - Series IIB - Mechanics-Physics-Chemistry-Astronomy, 1997. Summary.
———
Read the Norwegian version of this article at forskning.no
See more content from the University of Oslo:
-
Where humans outshine AI: “There's something hopeful in these findings”
-
Why we need a national space weather forecast
-
Mainland Europe’s largest glacier may be halved by 2100
-
AI makes fake news more credible
-
What do our brains learn from surprises?
-
"A photograph is not automatically either true or false. It's a rhetorical device"





































