An article from Norwegian SciTech News at SINTEF

Capturing hormone mimics with particles
Not only do they represent a serious threat to our health, hormone mimics accumulate in water and pollute the environment. Now there is a way of capturing them.
Denne artikkelen er over ti år gammel og kan inneholde utdatert informasjon.
In a laboratory in Trondheim, Norway, researchers have managed to create minute particles with some very desirable properties, such as the ability to capture and break down any hormone mimics that have ended up in our waste water.
“We know that hormone mimics are harmful to us, and that they don’t break down naturally,” says Per Stenstad.
Hormone mimics can be found in cleaning products, cosmetics, plastics, paint and electronics.
“They do not go away, but accumulate in waste water and soil,” Stenstad says.
Stenstad is a SINTEF researcher and chemist with a particular interest in applying the laws of physics at particle level. This is why he is sitting with us today to explain what they are creating in the lab behind us. He has brought along his sketch pad so that he can show us.
The technology that he and his colleagues are working with is so small that it cannot be seen with the naked eye. It is also very smart. Despite this, the researcher claims that the particle can be produced very easily – and what is more important, that it can be done cheaply and in large quantities.
It’s not easy to see the particle, since it measures no more than a few micrometres in diameter, but Stenstad can give us an idea:
Harmful effect on fertility
“Hormone mimics are tiny molecules that look something like this,” says Stenstad, drawing a ring-shaped structure.
“The problem is that our bodies cannot tell the difference between these and real hormones, which have roughly the same shape.”
The real hormones include testosterone and oestrogen, which play a key role in reproduction. Because the hormone mimics are so similar to these sex hormones, this causes a real problem.
“The problem occurs because the fake hormones are a perfect fit for a number of receptors in the body, particularly those in the reproductive organs. Once they are there, they block the receptors from receiving the real hormones that the body is actually looking for,” explains Stenstad, drawing two connecting pieces of a jigsaw puzzle.
As a solution to this problem, Stenstad and his colleagues have managed to produce a kind of ‘decoy’ – a particle that not only attracts hormone mimics but also breaks them down into harmless compounds. The particles are extremely porous. Just ten grammes of these particles have a surface area the size of a football pitch. With such a huge surface area, the capacity of the particles to capture hormone mimics is enormous.
Particle construction set
What makes the porous particles able to capture the harmful substances is the fact that their entire surface has been coated with the enzyme laccase. This enzyme is very effective in breaking down hormone mimics. It works by capturing the fake hormones and breaking them down into harmless compounds, which it then releases. The enzyme is then ready for another session. They are biocatalysts and can be used again and again.
“Laccase is extremely well suited to breaking down hormone mimics, but the problem is that if you just add laccase to waste water, it literally goes down the drain without having any effect. To get around this problem, we attach the enzyme to these particles,” explains Stenstad.
Advanced water filter
The particles created in SINTEF’s laboratories have been given properties that enable them not only to provide a ‘home’ for the efficient enzyme, but also to attract hormone mimics. The pores in the particle are large enough to allow the substance to be treated by the enzyme deep within the particle, and also to allow the harmless substances to be removed.
“We put the particles into a kind of membrane basket, through which the waste water passes. Since the enzymes are attached to the particles, they will not be washed away and will be active over a long period,” says Stenstad.
Big surface area – large effect
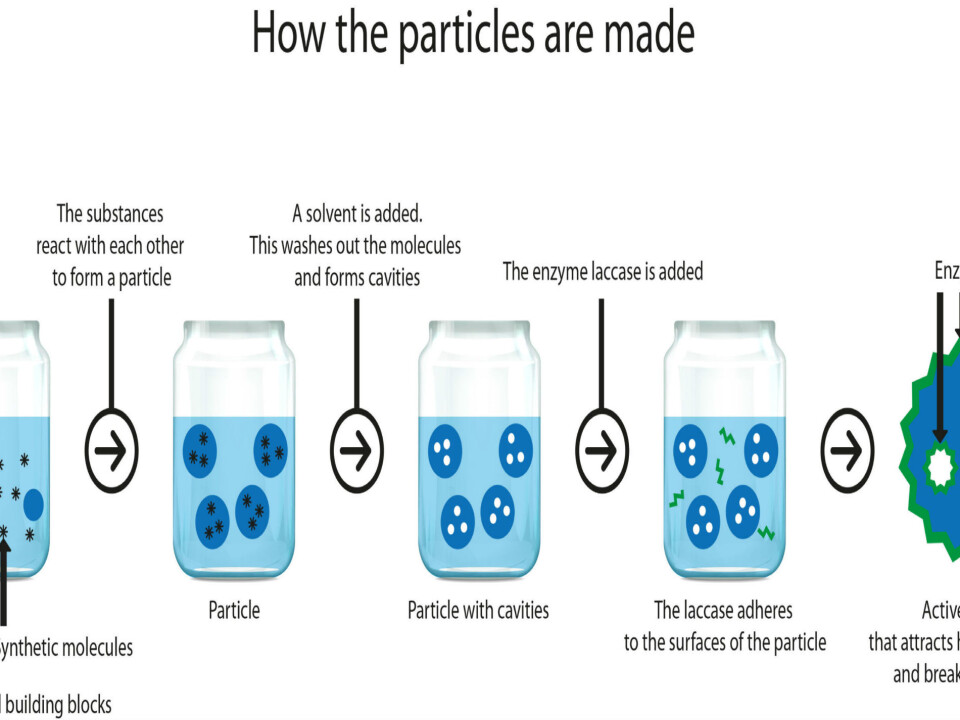
The SINTEF researchers construct the particles from what are known as monomers, which are chemical building blocks that react with each other and form a kind of network. In order to create porous particles, large molecules are added to the mix, and these act as stencils. They are not involved in the reaction and can be removed afterwards.
“What happens is that the molecules leave a number of small holes behind them, and it is these that make the particle porous. And it is this porosity that makes the particle so effective, because the cavities help to give it such a large surface area,” explains Stenstad, drawing something that looks rather like a very fatty slice of salami.
Finally, the particle is treated with a chemical substance that reacts with the active enzyme laccase. When that happens, the enzyme becomes anchored to the surface of the particles, so that it stays there and remains active for a long time.
“We can do all of this in a test tube, although if we want large quantities of the particle, the process can be scaled up into an enormous tank,” says Stenstad.
Many areas of application
The technology has also been tested in a pilot facility, and the results were exactly as the researchers had expected. But if the technology is to achieve its full potential, it has to get out of the laboratory and into the water treatment systems.
“The potential applications for this technology are huge, because we have managed to attach active enzymes to particles with an enormous surface area. For example, the salmon farming industry is extremely concerned about water quality, so we would like to work with them to test the particles”, says Stenstad.
“The method can also be used to capture CO2 from gas, so we hope that this will make more people interested in adopting our approach”, he adds.
The project is an EU project as part of ERA-NET (MATERA), with participants from Norway, Belgium and Switzerland.