THIS CONTENT IS BROUGHT TO YOU BY SINTEF - read more
Where kelp is being turned into lab-grown meat
Researchers are growing the food of the future in this lab: meat made using kelp instead of animal-based ingredients.

It's possible to grow meat in a lab. This means you can make a burger without slaughtering a cow or bull. But currently, it's still wildly expensive.
Lab-grown – or cultured – meat requires a lot of space. It often requires blood from aborted calves and microscopic, inedible beads. But this could change if senior research scientist Hanna Haslene-Hox and her colleagues achieve their goal.
Creating animal proteins – without animals
“How're we going to make animal proteins in a way that does not involve animals at all, or to a much lesser extent?” says Haslene-Hox, outlining the big question that SINTEF and Nofima are tackling together.
To that end, the researchers are using kelp – the largest subgroup of seaweed – as well as other seaweed species and plant waste instead of blood and synthetic material.
But growing proteins in the lab is not enough. It also needs to be affordable and possible to grow on a large scale.
“The first lab-grown burger was produced in 2013. It cost 250,000 euros,” says Haslene-Hox.
Need for a better suspension culture
The muscle cells the research team is growing need something to attach to in the suspension culture.
“We do that really well in culture bottles where the cells can grow in a super-thin layer on the plastic. But if you’re going to grow enough cells for a kilogram of meat that way, you would need 700 square metres of bottles. It’s not very practical,” says Haslene-Hox.
She adds that 700 square metres is equivalent to 10 average-size Norwegian apartments.
The key is a thicker layer
Currently, the cells grow in a layer thinner than a hundredth of a millimetre. To make lab-grown meat mainstream, researchers first have to enable the cells to grow upwards.
“Instead of growing only on a flat surface, they could grow on tiny microcarrier beads. Then we could fill up a tank with beads that have cells on them. That way you create a much larger surface area for the cells to grow on,” she explains.
This approach is already being used to some extent today. Researchers use spherical microcarriers made from dextran, a long chain of sugar molecules called a polysaccharide.
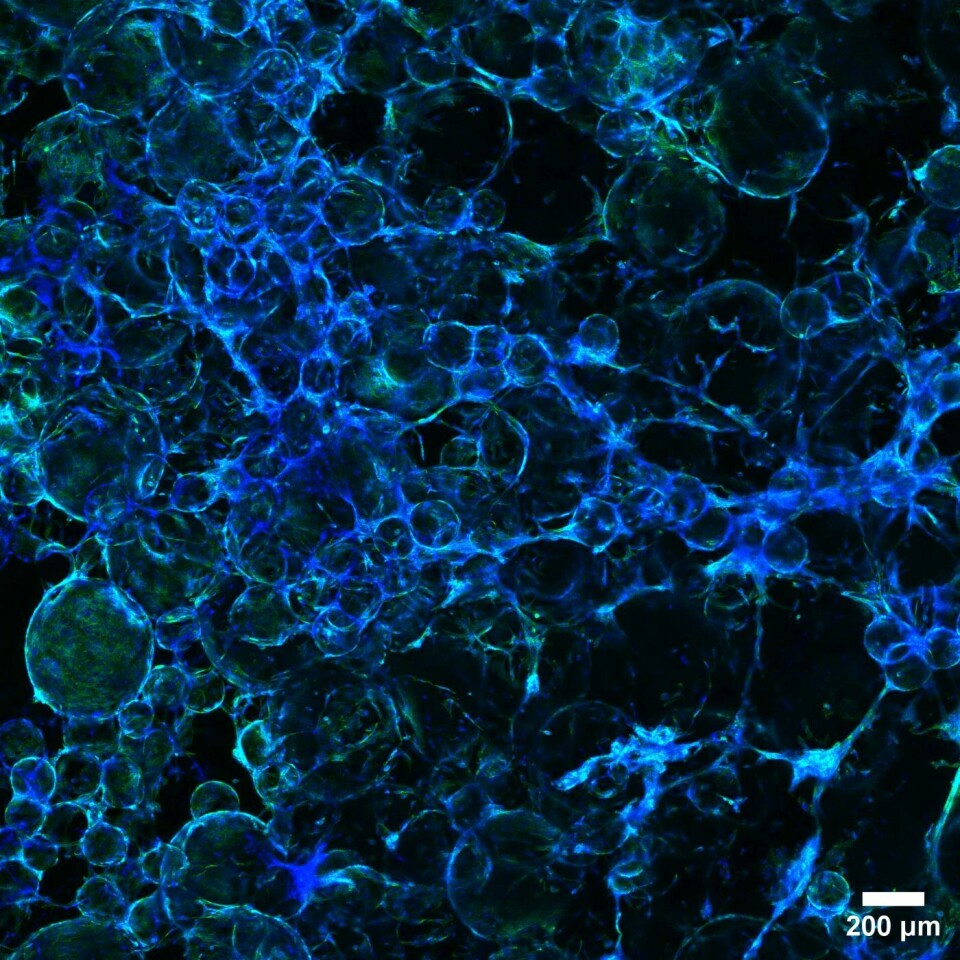
From synthetics to edible seaweed
“But you can’t eat these inedible beads. What you have to do if you’re going to make a steak or a burger is tear the cells loose from the microcarriers after they’ve grown sufficiently. This is a resource-intensive operation, and a lot of cells don’t tolerate it, so they die during the treatment. This generates a lot of waste in the process,” says Haslene-Hox.
SINTEF and Nofima aim to replace these dextran beads with natural materials.
“We’re trying to take bioresources that are left over from producing other things or that we have a lot of, like seaweed and kelp. Then we use them to make microbeads that the cells can grow on and that can then become part of the food," she says.
Haslene-Hox says that their project is about making microcarriers that cells can grow on and scaling that up in a big stirred-suspension tank.
Food scraps could also help
The next challenge is feeding the cells. Currently, they are often fed foetal calf serum, which is blood harvested from aborted calves.
“If you’re going to make a product that doesn't depend on animal husbandry, it’s stupid to use blood. Plus it’s expensive, difficult to obtain, has varying quality, and you can’t put it in people’s food. We have to try to find resources that we can use to feed those cells so that we don’t have to use that kind of serum,” she says.
The cells need both a liquid to float in and the microcarriers to attach to.

“We believe that we should be able to make both of these things from bioresources that are available in Norway,” says Haslene-Hox.
She lists some possibilities: seaweed, kelp, waste from vegetables and plant-based processing, waste from other food industries like salmon farming, eggshells, skin, and offal from chickens and cattle.
Can eggshells be useful?
One research partner, Norilia, works with eggshells, feathers, and skin. The thin membrane inside an eggshell is part of the chick’s embryo sac. It supports cells growth so well that it's even used to heal wounds.
“We’ve looked at whether muscle cells are able to attach to particles from eggshell membranes or whether we can mix them with alginate to get the cells to attach,” she says.
So far, the team has found some materials that muscle cells seem to really like to grow on. The next step is to use those microbeads to scale up the process.
Haslene-Hox poses some final questions: “What will happen if we start stirring this mixture? Will the cells stay attached to the bead surface?”
More content from SINTEF:
-
300 meals can be saved from this kitchen – every month
-
Saving seagrass and French oysters: New solutions give new life to Europe's coastal areas
-
What does ultra-processed food do to our gut flora?
-
This new device can make it cheaper to heat your home
-
Propellers that rotate in opposite directions can be good news for large ships
-
How Svalbard is becoming a living lab for marine restoration






































