An article from University of Oslo

Researchers are testing new method against antibiotic resistant bacteria
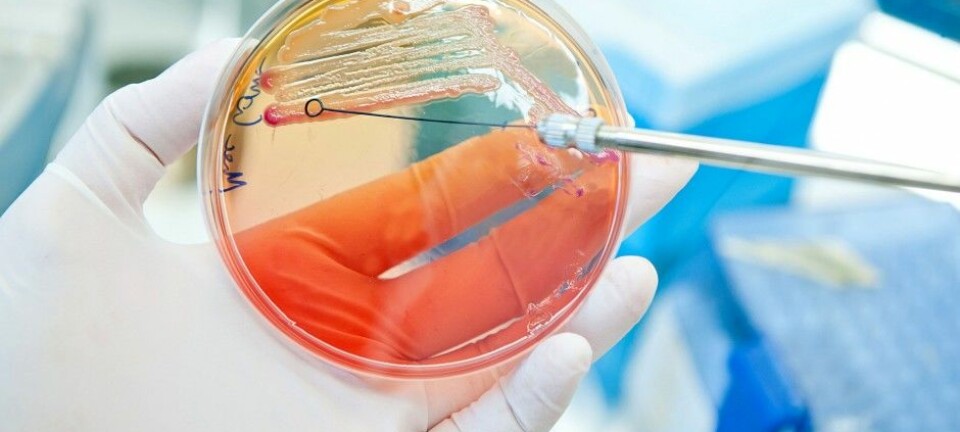
Norwegian researchers are experimenting with a new compound that could disarm multi-resistant bacteria.
Denne artikkelen er over ti år gammel og kan inneholde utdatert informasjon.
Ever since the 1940s when the antibiotic penicillin was introduced, we have used antibiotics to cure bacterial infections. Now, a growing number of bacteria are becoming resistant to antibiotics, rendering these drugs ineffective for treatment.
"Penicillin is one of the greatest revolutions in medical history, but all antibiotics sooner or later cause development of resistance. For this reason, the pharmaceutical industry has been reluctant to put any effort into new products, and no genuinely new antibiotics have been launched for the last twenty years. We are therefore entering a post-antibiotic era, where we are back to the time before there were any antibiotics that could cure bacterial infections," Pål Rongved explains.
He is professor at the School of Pharmacy at the University of Oslo (UiO), and is working on antibiotic resistance.
A molecule upsets the zinc balance
Rongved and his research group are seeking to identify new agents that can be used in the struggle against antibiotic-resistant bacteria. Their approach is to use a molecule, a so-called zinc chelator, that can selectively bind the metal zinc and upset the zinc balance in the bacteria.
Beta-lactam-type antibiotics such as penicillin have increasingly become ineffective because an enzyme in the bacteria – beta-lactamase – destroys the antimicrobial activity of the antibiotic.
Rongved explains that their zinc chelators upset a sub-group of lactamases – metallo-beta-lactamases – that depend on zinc, thus rendering the enzymes unable to harm the effect of the antibiotic.
"Our zinc chelator is what we refer to as an adjuvant. This means that it needs to be ingested along with another molecule, in this case a beta-lactam antibiotic. Our chelator has a large reinforcement effect on commercial antibiotics when tested on various bacteria – including those that in principle are resistant to the antibiotic."
Long experience with metal compounds
"I have undertaken research on metal compounds for 25 years, including many years in the pharmaceutical industry. In 2009, we tried to determine whether we could inhibit cancer cells by using zinc chelators to upset their zinc balance," Rongved says.
He goes on to say that their entry into the field of antibiotic resistance and the use of zinc chelators on bacteria came about when they became aware of Ørjan Samuelsen and his colleagues at the Competence centre for detection of antibiotic resistance (K-res) at the University Hospital of North Norway (UNN), who studied the metallo-beta-lactamases that bacteria use to destroy antibiotics – and that these contained two zinc atoms.
"When we tested our zinc chelator on the bacteria, we were surprised to find that it had antimicrobial properties. The chelator upset the zinc balance in the bacteria, thus inhibiting the metallo-beta- lactamase."
Further tests are needed
Bacteria and our cells share the need for an optimal zinc balance in order to function normally. Humans have approximately 6000 enzymes that depend on zinc. It is therefore also extremely important to make sure that the zinc chelator will not harm our own cells.
The researchers have equipped the zinc chelator with a target-seeking component causing it to concentrate mainly on bacteria and less on human cells. They have now started to test the zinc chelator on human cells in the laboratory, and things are looking good so far. Soon they will start tests on a larger scale.
-------------------------------------
Read the Norwegian version of this article at forskning.no
Translated by: Akasie