THIS ARTICLE/PRESS RELEASE IS PAID FOR AND PRESENTED BY NTNU Norwegian University of Science and Technology - read more

Using real neural networks to pinpoint the start of brain disease
Researchers at NTNU are studying brain cells in the lab to investigate the foggy beginnings of diseases like Parkinson’s and Alzheimer’s.
When the symptoms of neurodegenerative diseases like Parkinson’s become clear enough to make a diagnosis, there have already been significant changes in a person’s brain. That’s why researchers believe that finding a way to identify this turning point could be the key to better treatments.
“In theory, if you can pinpoint the onset of the disease, you might be able to stop it or reverse some of its effects,” says Ioanna Sandvig, co-leader of the integrative neuroscience group at NTNU’s Department of Neuromedicine and Movement Science. “By the time you actually have very strong indications that something is wrong, then it’s a bit too late.”

Sandvig and her colleagues are growing interconnected brain cells in the lab, to study how these neural networks evolve and what happens when things go wrong. The research, partly funded by NTNU’s nanoscience initiative, 'NTNU Nano', could help to pinpoint the very beginnings of neurodegenerative diseases.
Chip that mimics connectivity
Each neural network contains bundles of neurons – the cells that carry messages in our brains – housed in a microfluidic chip, the prototype of which was designed by Rosanne van de Wijdeven during her PhD at NTNU. These nodes are connected by tunnels in the chip through which axons – the wire-like protrusions of a neuron – can grow, but the main body of a neuron cannot.
By connecting three nodes together the researchers can mimic the connectivity inside the human brain. But Sandvig is keen to stress that these neural networks are not in any way real brains. “We don’t have brains in the lab,” she says. “But we do have networks that are representative, and are very malleable to the perturbations we want to introduce.”
The chips contain microelectrode-arrays made in NTNU’s NanoLab that enable the researchers to measure the electrical activity of the network and gain insight into how signals are passing between the neurons.
Confused neurons
In one recent study, led by Vibeke Devold Valderhaug and detailed in a paper uploaded to the pre-print server bioRxiv, the researchers cultivated two groups of neural networks in parallel, one of which housed neurons with a gene mutation that has been linked to Parkinson’s disease known as LRRK2 G2019S. They saw that, in the network with the mutation, neurons grew and formed connections in a markedly different way, and displayed different electrical activity, to the healthy network.
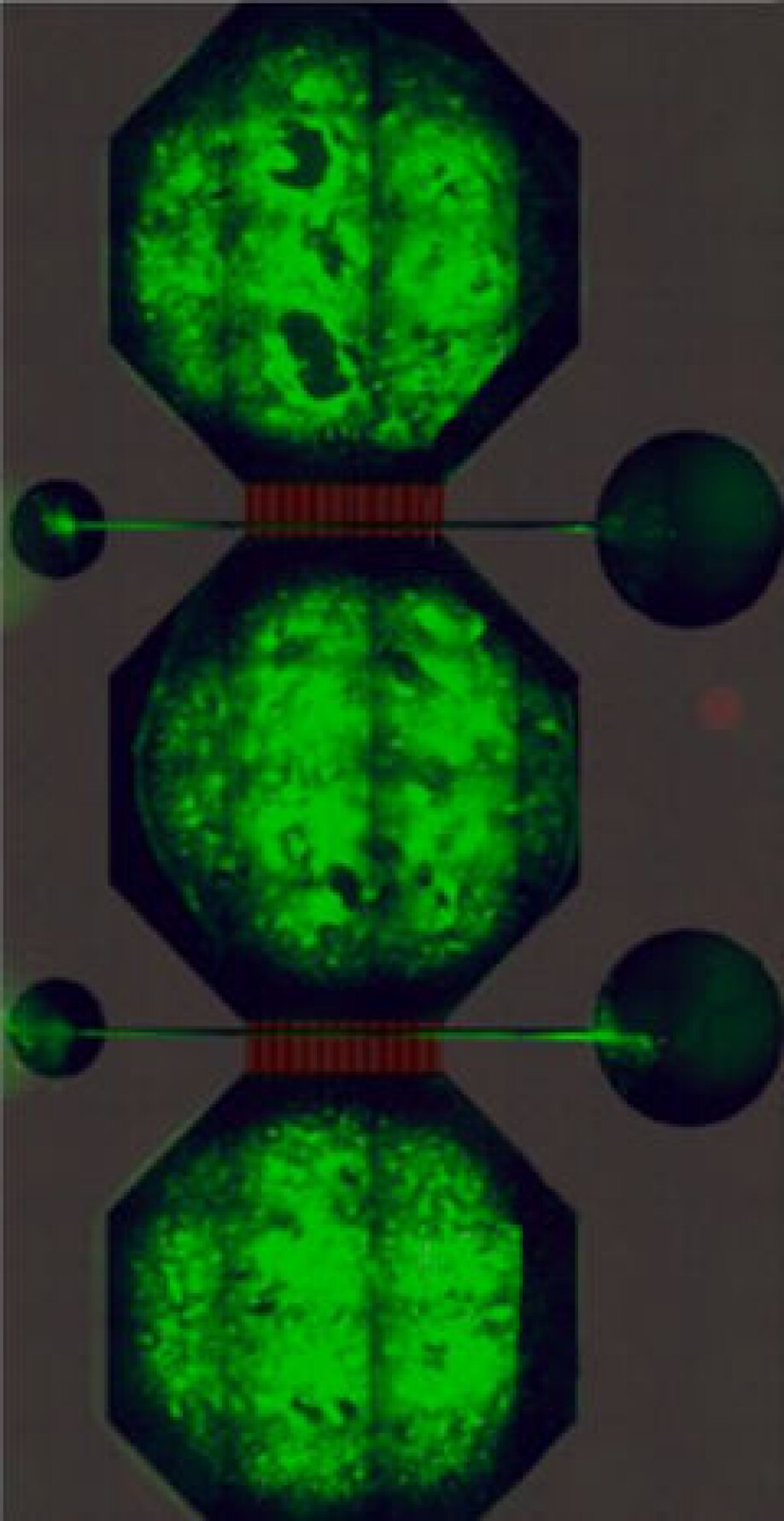
Neurons typically navigate their environment in a very specific manner, says Sandvig, but in the network with the mutation the cells didn’t have the kind of directionality you would expect – they seemed confused. “The LRRK2 mutated networks seem to have some kind of aberrant growth,” says Sandvig. “They seem to interpret exactly the same cues – because it’s the same substrate – in a totally different way than the healthy network.”
As well as those findings, Sandvig was pleased to see how clearly the subtle changes showed up in the team’s experimental set up. “What was surprising was how well we could pick it up with this interface,” she says. The interface also allowed the researchers to look at the connectivity within the nodes as well as between them.
Different brain diseases with shared basic characteristics
Studying these brain changes in a neural network has advantages over studying them in animals, though each method can inform the other. “You can have these snapshots of changes in the structure and function of the network much more easily than in animal models,” says Sandvig.
With neural networks, it’s also possible to take cells from the same individual and derive them in two separate ways to compare how the networks evolve when age-related effects are removed. In fact, one of the follow up projects Sandvig is developing with integrative neuroscience group co-leader Axel Sandvig alongside colleagues at the Kavli Institute for Systems Neuroscience does exactly this for Alzheimer’s disease. “Diseases like Parkinson’s, ALS, Alzheimer’s are all different, but they share some very fundamental characteristics,” she says.
By providing new insights into how our brains change in the early days of neurodegenerative disease, real life neural networks could set us on a path to new understanding, and perhaps, eventually, even new treatments.
Vibeke Devold Valderhaug et.al.: Structural and functional alterations associated with the LRRK2 G2019S mutation revealed in structured human neural networks. BioRxiv preprint, 2020.
See more content from NTNU:
-
Fish farming is least harmful to the seabed in the north
-
Study: Centralising hospitals has reduced birth mortality
-
Early testing of schoolchildren: “We found absolutely no effect”
-
This determines whether your income level rises or falls
-
Why is nothing being done about the destruction of nature?“We hand over the data, but then it stops there"
-
Researchers now know more about why quick clay is so unstable





































