An article from University of Oslo
Nerve fibres’ secrets revealed
Nerve fibres, which transmit impulses from neurons, play a key role in the nervous system. Until now, no-one knew how they formed.
Denne artikkelen er over ti år gammel og kan inneholde utdatert informasjon.
Every single impulse in your body is controlled by your nervous system. Sensations such as taste, sight, hearing and touch travel to the brain, while movement impulses travel the other way to the muscles.
The nervous system is made up of neurons and glial cells. Neurons are responsible for the actual transmission of impulses, while glial cells protect and support neurons in many different ways.
Nerve fibres enable neurons to connect with one another. They help to send signals to and from the brain.
Researchers at the University of Oslo have now discovered how these nerve fibres form.

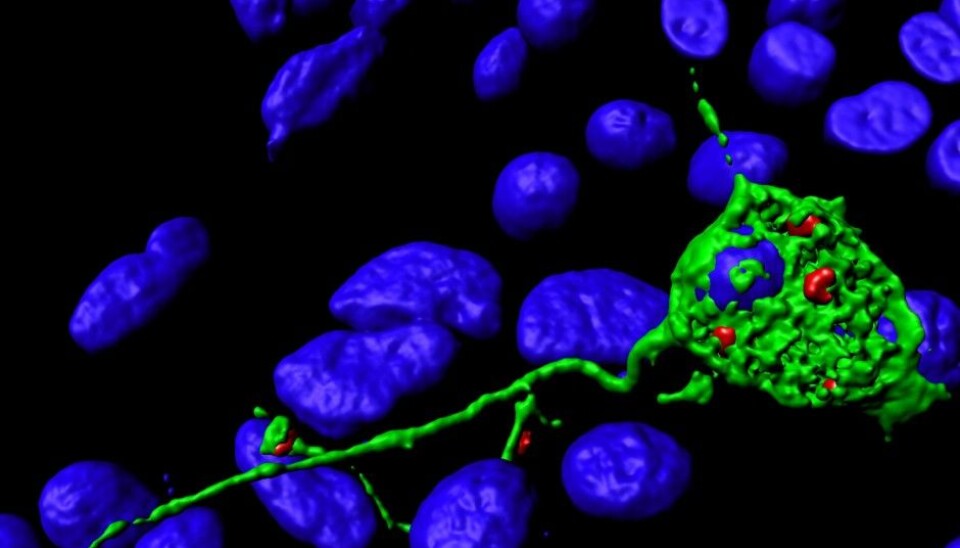
Nerve fibres are formed when small vesicles inside the neuron, called endosomes, merge with the cell membrane. The cell membrane is the cell's outer layer.
The discovery was made during microscopy studies at the Centre for Cancer Biomedicine (CCB). CCB is a centre of excellence at the University of Oslo (UiO) and Oslo University Hospital.
Faulty proteins lead to disease
Professor Harald A. Stenmark explains:

“Our research uncovered a group of proteins that bring about the process in which vesicles fuse with the cell membrane. It turns out that several of the proteins were already known to us, as so-called paraparesis proteins.”
Faulty versions of these proteins cause the rare disease hereditary spastic paraparesis, in which the legs become weaker and weaker.
Hereditary spastic paraparesis occurs when the longest nerve fibres in the body, those that go down to the feet, become damaged over time.
“We don’t know exactly how faulty paraparesis proteins lead to damage to nerve fibres.
Our hypothesis is that the nerve fibres require continual maintenance via a process in which small vesicles, endosomes, are carried out to their tips and fuse with the cell membrane. If this mechanism is disrupted, the result may be damage to the nerve fibre,” says Stenmark.
Cell transport system
Neuronal vesicles that come close to the cell membrane are able to fuse with it. The researchers have uncovered a role for a type of motor protein called Kinesin-1, which transports vesicles to the cell membrane. The transport takes place along structures called microtubules, which can be likened to train tracks inside the cell.
“The vesicles themselves form when part of the cell membrane bends inwards and buds off. They do this to take up nutrients from outside the cell, or to bring proteins from the cell membrane inside the cell.
The vesicles can either make their way to other vesicles and fuse with them, or they can fuse with the cell membrane.
It’s the latter process that we’ve been studying,” says Camilla Raiborg.
Proteins and microscopy
Stenmark explains that the discovery was made in two ways:
“We increased or decreased the levels of various proteins inside the cells. The main proteins we have been studying are called Protrudin, Kinesin-1 and FYCO1.”
Kinesin-1 is a motor protein that transports vesicles to the cell’s exterior. FYCO1 connects Kinesin-1 to vesicles. Protrudin ensures that Kinesin-1 becomes attached to FYCO1.
“We also studied these proteins inside the cell with the aid of advanced microscopy. We performed a lot of microscopy in living cells, as this meant that we could study the formation of nerve fibres directly,” says Stenmark.
Could explain the spread of cancer
The research group has previously studied ‘receiver proteins’, or receptors. These are proteins that are able to pick up signals from other cells.
Camilla Raiborg explains further:
“The signals usually take the form of small proteins that bind to the receptors. As a result of the binding, the signals are transmitted by the receptors and enter the cell inside small vesicles.
The signals are often instructions for the cell to divide.
Since cancer involves uncontrolled cell division, it goes without saying that it is undesirable for cells to have too many of these receptors.
It was while studying these receptors and vesicles that we discovered that the vesicles can also be used to produce outgrowths from the cells.”
The researchers believe that cancer cells may use a mechanism involving such outgrowths to enable them to spread.
“The theory is that cancer cells produce long outgrowths, not unlike nerve fibres. At the tips of these outgrowths, vesicles from inside the cell fuse with the plasma membrane,” says Stenmark.
In that way, the contents of the cancer cells are able to spread to the surrounding tissue, partly as a result of enzymes breaking down tissue barriers. Once the barrier has been breached and the cells have established themselves, the cancer has spread.
“If the theory holds true, it may open up new possibilities for preventing cancer from spreading,” concludes Harald A. Stenmark.

































