An article from University of Oslo
Immune cells from healthy people pulverise cancer
Immune cells from healthy individuals could be the new immune cure for cancer. This treatment can kill cancer cells without destroying neighbouring cells.
Denne artikkelen er over ti år gammel og kan inneholde utdatert informasjon.
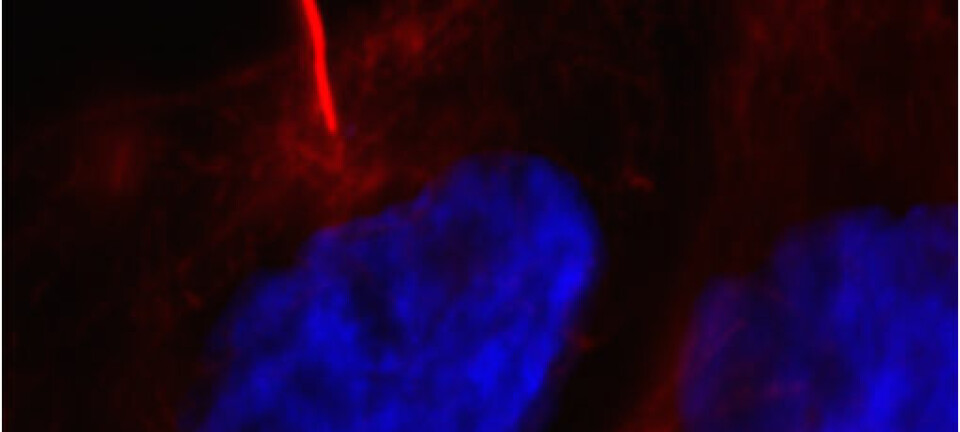
“Our vaccines are based on stimulating the patient’s own defence system to attack the tumour,” says Professor Johanna Olweus at the Immunology Institute at the University of Oslo. Together with her research team, she has found a new method of using the immune system to attack cancer.
“We have found a niche that few other people are aware of," says Olweus. "In order to achieve effective treatment the immune system must react strongly. This is difficult with the patient’s own immune system.”
Instead of making a vaccine that builds up the patient’s own immune system, the vaccine utilises a strong immune response from healthy individuals.
“Our studies show that the healthy immune cells attack and kill the cancer cells very effectively,” she says.
Own immune cells offer poor resistance to cancer

In order to understand the innovation, it is first necessary to understand why it has been so difficult to produce a vaccine against cancer.
Vaccination against infectious diseases is one of the greatest advances in the history of medicine. The immune system recognises a virus or bacteria as dangerous and foreign. When we vaccinate against a virus for example, a message is sent to the foot soldiers, the T cells, so that they are prepared. Then, any later viral infection can be knocked out by the immune system so quickly that we do not notice.
“However, we have not been able to successfully transfer this technique to cancer,” says the researcher.
Once the cancer has gained a foothold, it lives a relatively peaceful co-existence with the immune system, even though it would be desirable for the immune system to react aggressively.
Olweus believes this peaceful co-existence can be explained from an evolutionary perspective:
"The existence of the human race has always been dependent on an immune system that defeats infections. But in contrast to infections, cancer generally affects people after they have had children, when survival of the human race is no longer dependent on cancer being defeated by the immune system."
Immune cells commit suicide
Neither is it enough that immune cells identify cancer cells as foreign. Cancer cells must also be recognised as being dangerous. Unfortunately, cancer cells do not give enough danger signals because they only cause slight inflammation. Inflammation is important if the immune system is to react.
“A cancer cell must be both foreign and somewhat dangerous if the T cells are to react," she explains. "When the T cells do not recognise the cancer cells as dangerous, the T-cells kill themselves. This happens primarily with the T cells which could have given the most effective response.”
The explanation is that our immune system tries to protect us against over-reaction to our own tissues. Over-reaction can cause autoimmune diseases such as arthritis and multiple sclerosis.
And as if this wasn’t enough, the cancer cells have the abominable property of excreting substances that inhibit the surviving T cells.
Moreover, most of today’s vaccines aim at triggering an immune response against proteins that are present in higher numbers in cancer cells than in normal cells.
The problem is: these are normal proteins that are not normally recognised as foreign, even though there may be a particularly large number of them present in cancer cells.
Difficult mutations
A cancer cell can have hundreds of mutations. A mutation is a change in the DNA strand. These mutations can be recognised as foreign by the T cells.
The problem is that it is very difficult to find the mutations that are common to all patients with one particular type of cancer.
Mutations in cancer cells are generally specific for the individual patient, which makes it difficult to know what to target.
However, if it is possible to direct many 'weak' T cell responses to a large number of mutations, this could possibly have an effect. This may be the explanation why, in trials on treatment of melanomas, antibodies that remove the 'brake' for all types of T cells appear to have a promising effect. But this form of treatment is highly risky because the immune system can run "out of control, explains Olweus.
Immune response from healthy people
Today two types of immunotherapy are used as part of the standard treatment for cancer. These are based on immune responses that are produced outside the patient.
When you transfer an immune response to a patient, it’s able to function independently of the patient’s own weakened immune system.
This has resulted in a number of success stories.
The first type of treatment uses therapeutic antibodies that are made by vaccinating animals with human cells. The antibodies recognise the proteins that are only found on a certain cell type. This treatment is particularly effective in lymphatic cancer, although the antibodies also kill a certain type of healthy immune cells known as B cells. These B cells are an important part of the immune system.
The second type of treatment is a bone marrow transplant from healthy individuals to patients with leukaemia or lymphatic cancer. This treatment is highly challenging and can be the patient’s only chance of survival.
The transplanted bone marrow contains both blood stem cells and healthy T cells from the donor. These T cells can attack the cancer cells and in the best case cure the patient.
In contrast to the patient’s own T cells, which have been significantly weakened by the disease, the new and healthy T cells from the donor have not been exposed to 'tolerance' over a long period of time. Therefore the T cells do not commit suicide. They react instantly to the foreign immune cells. The explanation is that the chemotherapy and radiotherapy have triggered the inflammation and the danger signals.
“The T cells will be able to recognise the cancer cells as both foreign and dangerous and attack them," says the researcher.
"The treatment is effective, but is also so dangerous that it is normally only given to patients younger than 60 who are in good health. The side effects are significant. In three of four cases, the added T cells also attack normal cells in the skin, liver and intestines. In the worst case, the patient can die from the treatment.”
Can remove undesired effects
The research group led by Olweus has managed to produce a method that distinguishes between desired and undesired effects.
The results have been published in a number of internationally-recognised journals such as Leukemia.
“Our method is now being used to produce T cells that kill certain types of cancer cells,” says Olweus.
In order to produce the desired T cells, they use cells from healthy volunteers. The T cells target a certain protein.
“Then we can use the same principle as that used so successfully in antibody therapy. We target the attack at a given cell type by making these T cells recognise parts of a protein that is only found in this cell type.
The T cells can then kill all cells that contain this protein, both healthy and sick. Normally, T cells do not react to these normal proteins.
“The reason that we can get T cells to recognise such proteins as foreign is that we combine the T cells with foreign tissue type molecules," she says.
Tissue type molecules are found in nearly all cells. They are located on the surface and tell the immune system what is happening in the cell. Thus immune cells, just like the T cells, can receive a message that there is something foreign in the cell that must be killed.
If a patient has a type of lymphatic cancer called B cell cancer, prostate cancer or ovarian cancer, the patient can tolerate that the treatment also kills the healthy cells.
It is fully possible to continue living without B cells, a prostate or ovaries.
Prize-winning target-seeking missile
However, Olweus wants to take it a step further:
“T cells kill in a different way to antibodies or chemotherapy," she says.
"T cells can thus be highly effective when antibody treatment or chemotherapy does not work. But all treatments involving cells have a high resource consumption. Another goal for our immunotherapy is therefore to use the T cell receptors that work as 'Target-seeking missiles'."
Olweus and her colleagues have found a method to isolate the DNA code for the 'target-seeking missiles' and produce them as soluble molecules. This means the treatment can be administered intravenously. A patent has been applied for, and last year the method was awarded the annual innovation prize from the Innovation company Invent2 from the University of Oslo and the South-Eastern Norway Regional Health Authority.
T cells have the potential to be a far better attack weapon than antibodies. Treatment with antibodies primarily prolongs life expectancy. Few of them cure.
“Antibodies have a substantial limitation. They only recognise proteins on the cell surface. In contrast, T cells also recognise proteins inside the cells. The vast majority of proteins are found only inside the cells. The new therapy can be directed at the proteins inside the cells that are important for the survival of the cancer cells. This can be an important innovation in the battle against cancer."
Combined treatment
Johanna Olweus expects that this treatment can be given in combination with antibodies, chemotherapy and radiotherapy.
In order to determine which proteins the treatment has to attack, the research team has mined databases which compare protein collections in cancer cells and organs from thousands of patients.
“If there is a high concentration of one protein in the organ in which the cancer originates and the protein is practically absent from the other normal organs, we can use this protein as a target for the T cells," she says.
Hope to eradicate cancer
The treatment could solve one of today’s greatest problems in cancer therapies. After chemotherapy and radiotherapy, loose cancer cells continue to circulate around the body.
“This immune therapy offers the possibility to also destroy these cancer cells, without harming neighbouring cells. This is important. Our hope for the future is that cancer can be eradicated for good, but we must take this step by step. We anticipate that this treatment can be tailored for all types of cancers in organs that are not essential for us to live, such as the prostate, ovaries and breasts.
"We also anticipate that the treatment can also work against cancer in those organs which today can be transplanted, such as blood, kidneys and liver. The hope is that our new treatment can be trialled on patients within a few years,” concludes Johanna Olweus.