An article from Norwegian SciTech News at SINTEF
Digital models of individual patients’ hearts help optimise surgery
Every heart is unique. Cardiologists and engineers have teamed up to simulate the structure and blood flow of patients’ hearts using ultrasound and modern software. This can raise the success rate of surgical procedures.
Denne artikkelen er over ti år gammel og kan inneholde utdatert informasjon.
Ten per cent of today’s cardiovascular operations are unsuccessful. This lead to a search for technologies and tools that can reduce the risks involved, and clinicians at Norwegian university hospitals and SINTEF researchers now cooperate to find new solutions.
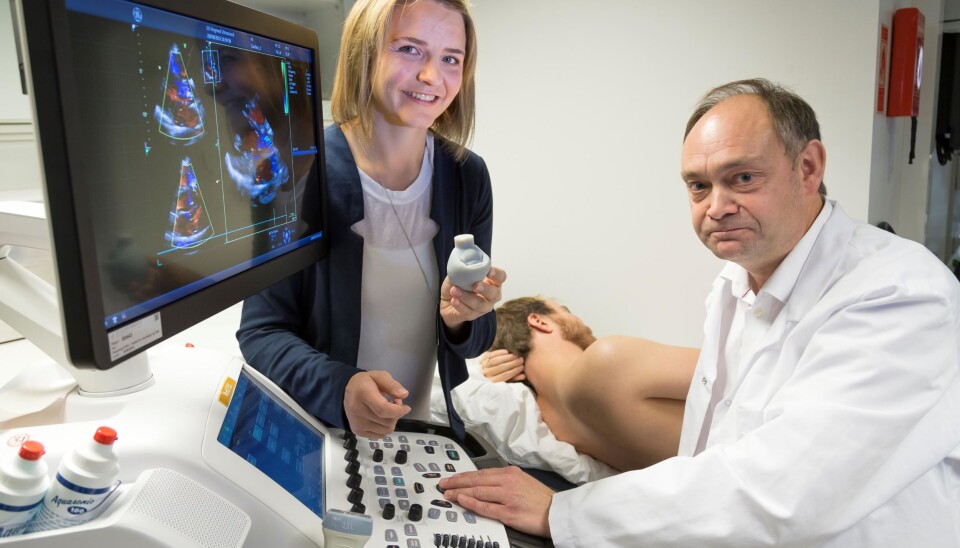
Research scientist Sigrid Kaarstad Dahl and her colleagues generate on-screen models of a patient’s beating heart, and simulating patient-specific blood flow patterns. This enables them to predict the effects of heart surgery.
“It means that we can simulate an operation in advance and say something about what procedures will be of most benefit to the patient in question”, says chief surgeon and cardiologist Stig Urheim, who works at Haukeland and Oslo University Hospitals.
Heart valve failure
Cardiovascular disease is currently the most common cause of death in the developed world, and also the most expensive for society as a whole. Moreover, the number of patients suffering from cardiovascular disease is expected to increase considerably in the years ahead.
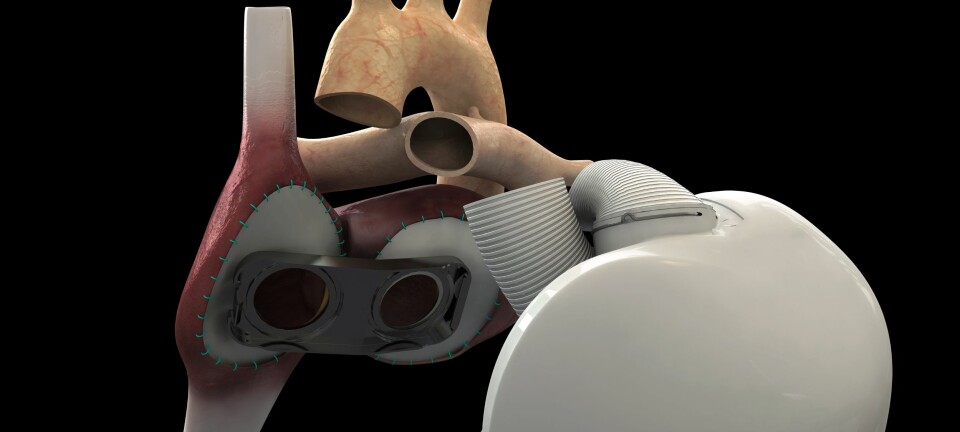
One of the causes of heart failure is heart valve malfunction, and current treatments involve attempts either to repair existing valves or implant prosthetics.
For any given patient, no-one can be sure in advance which of the many different treatment techniques will result in the best outcome. Since many valve repair procedures are unsuccessful, requiring a new operation, this means more hospital admittances, increased levels of medication, reduced quality of life and increased mortality among patients. And this in turn means longer waiting lists and increased public expenditure.
Hearts on screen
Dahl thought that this problem was so interesting that she took her doctoral degree at NTNU/Simula and wrote her thesis on the simulation of cardiac blood flow patterns. After she started work at SINTEF, the topic was matured and research became funded by the institute. Today a large group of researchers, engineers and clinicians from all over Norway are working on the project.
Stig Urheim is just one of them. He says that anatomy is unique to each individual patient.
“Our research tells us that blood flow across the valve between the atrium and the ventricle varies from patient to patient, depending on where the pulmonary veins empty into the atrium. Implanting a prosthetic valve may have a significant effect on the outcome of the operation”, he says.
Prosthetic valve surgery can alter blood flow patterns
Researchers use ultrasound or MR images to create a model of a patient’s heart. Dahl uses her own beating heart displayed on a screen as an example of what she’s talking about.
“The screen display enables us to use the simulation tool to take the differences between patients’ hearts into account prior to implanting prosthetic valves”, she says.
She shows us some of the simulations that illustrate blood flowing through her heart.
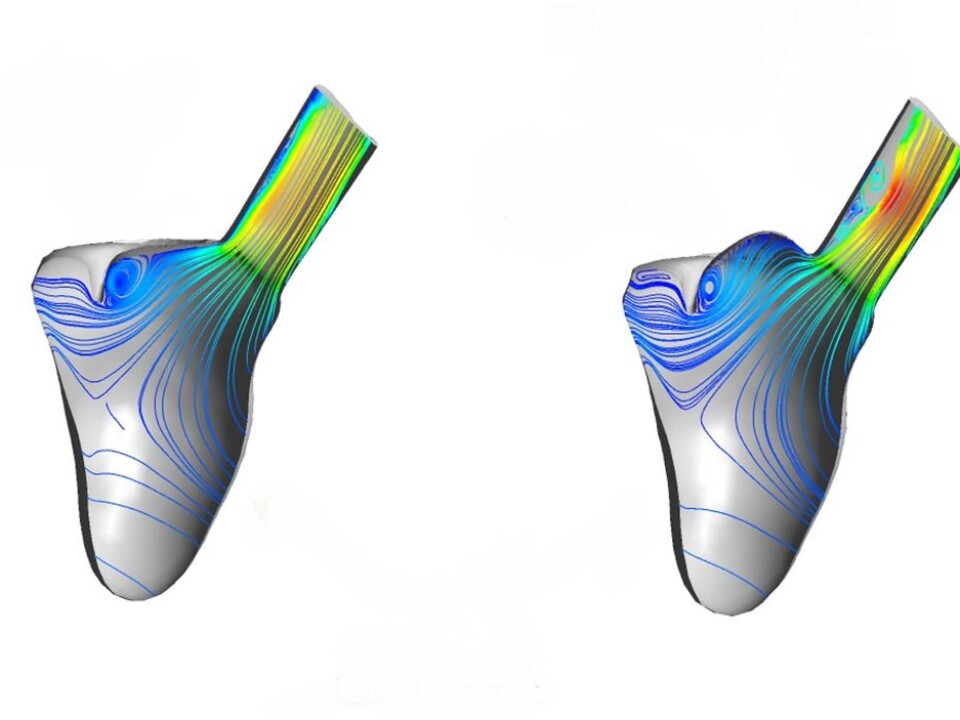
“The prosthetic valve is sewn in permanently where the malfunctioning valve was located. After implantation, you can see how the blood flow pattern changes, depending on its location and the particular anatomy of the heart in question”, she continues.
She displays the image of a cross-section taken immediately above the valve.
“For example, if I make a small alteration to the veins entering the ventricle here, and at the same time position the valve like this …” – Dahl uses the cursor to move the blood vessels up and down on the screen – “… you can see that the blood’s velocity profile across the valve changes – and with detrimental effects on the flow pattern”.
This may explain why some patients suffer from blood clots that develop in their heart valves, which is a life-threatening condition.
Detailed planning
Findings such as this have led researchers to envisage more systematic treatment programmes in which doctors, in addition to the information they currently obtain from ultrasound examinations, can obtain results from 3D cardiac blood flow simulations both before and after planned surgical procedures.
“If a valve has to be repaired, the simulation tool will provide the doctors with useful information”, says Dahl. “We have not had access to this type of detailed information before”, she says. Such information will help to prevent the development of adverse flow patterns that can lead to new cardiac disease and damage.
Dynamic hearts
Dahl tells us that blood flow simulations for different heart valve geometries is a hot topic globally, and that many research groups are working along the same lines as the Norwegian team. Currently, all groups are very much in the research phase, but researchers in Trondheim are occupying pole position.
“Some overseas groups are working with ‘rigid’ heart models, with no mobility built into the heart wall”, says Dahl. “We, on the other hand, are running simulations with dynamic heart models. And we’re employing ultrasound, while many others are using the more expensive and time-consuming MR and CT approaches. We are also fortunate in our project to be able to call on highly skilled doctors. This ensures that our progress is clinically relevant at all times”, she says.
Work is now focusing more intensely on the application of heart and valve models in close collaboration with Stig Urheim and Professor Bjørn Skallerud at NTNU. A research collaboration is being established with Northwestern University in Chicago, which is among the foremost cardiac valve treatment centres in the USA.
“There are a number of specific methods that we’re currently working on, but we must streamline our software in order to make it easier to get the technique established”, says Dahl. “First in clinical studies, and then in clinical practice”, she says.
-------------------------------------
Read the Norwegian version of this article at forskning.no