THIS CONTENT IS BROUGHT TO YOU BY University of Oslo - read more
These researchers want to turn CO2 into stone
Nature got there first: When CO2 reacts with volcanic rock, it transforms from a greenhouse gas into solid minerals.

We have to go back 55 million years. That was when Greenland and Norway began to drift apart, causing the Atlantic Ocean to open up. The Earth's crust between them became thinner and thinner, and enormous amounts of lava poured forth.
At the same time, the Earth suddenly became dramatically warmer. This chapter in Earth's climate history naturally interests geologists. In 2021, they sent a research ship out into the sea to collect samples from the seabed.
Some of the samples ended up with PhD candidate Marija Plahter Rosenqvist. She is not trying to figure out why it got so warm in the 'old days,' but rather how we can prevent it from suddenly becoming as warm now.
The researchers are investigating whether we can store CO2 (carbon dioxide) in the lava rock that was formed when the Atlantic Ocean came into being.
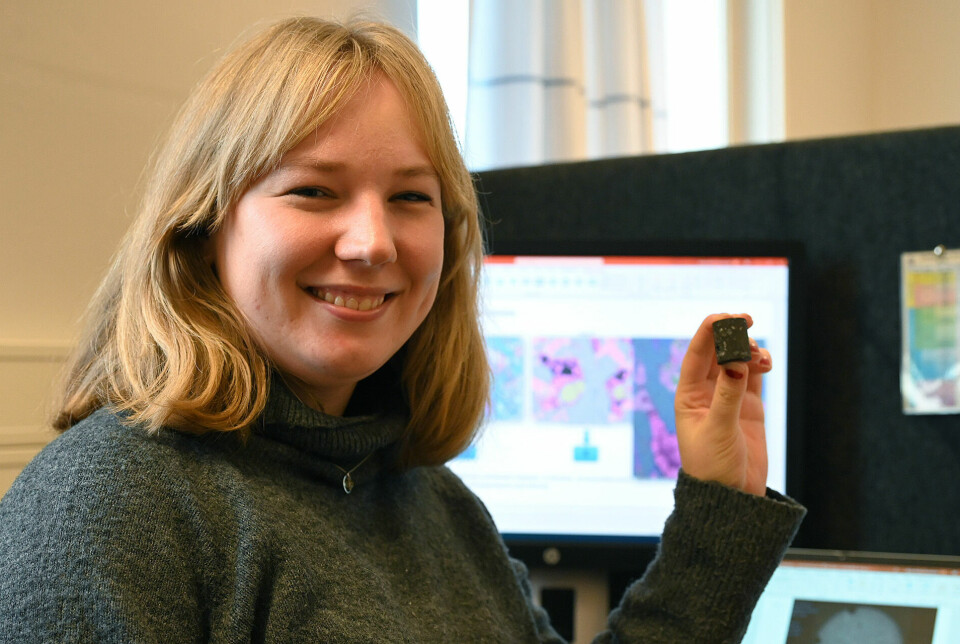
Rosenqvist's sample of dark grey rock has holes in some places, somewhat like a chocolate bar with air bubbles. In other parts, it appears that the holes are filled with a lighter type of rock.
CO2 in solid form has many advantages
The rock is basalt, the most common type of lava rock on Earth. The white spots are formed where CO2 has reacted with volcanic rock and transformed from gas into minerals.
Researchers at the University of Oslo's Njord Centre are studying how this process occurs naturally. The goal is for this to become part of the solution to the climate problem.
“Storing CO2 in solid form has many advantages. We don't need to monitor the reservoir over a long period since it doesn't leak,” says Rosenqvist.
The method also has great potential.
“More than 60 per cent of the Earth's surface is covered by basalt, so in theory, there is enough space for all the CO2 we want to dispose of,” she says.
The principle is not entirely untested. The Carbfix project in Iceland serves as an inspiration for researchers in Oslo. However, there is one major difference: The basalt used in the Iceland project is fresh and porous, whereas in the North Sea, it's 50 million years old.
“To understand whether we can do this in places other than Iceland, we need to conduct more studies,” says Rosenqvist.

The basalt from the North Sea can be found above sea level in the Faroe Islands
In the same office sits Rakul Maria Ingunardóttir Johannesen.
Together, they have been to the Faroe Islands equipped with drones, among other tools, to map the geology.
“The Faroe Islands are interesting because we find the same basalt here as we do out in the North Sea,” says Johannesen.
It might also be possible to store CO2 on land here.

“We already store CO2 in reservoirs in the North Sea, but that's in sandstone,” Johannesen explains.
“Basalt is very suitable because it quickly reacts with CO2 and forms solid minerals,” she adds.
A bit further down the corridor, we meet Paiman Shafabakhsh, who has almost been on a European tour with basalt samples.
What happens in the small pores of the rock?
While Johannesen studies geological formations and their fracturing, Shafabakhsh is focused on understanding what happens on a smaller scale, in the small pores of the rock.
“We inject CO2 and fluid into the rock samples and want to know what occurs within the rock during this process,” he says.
To achieve this, the researchers scan the rock with X-rays and neutrons.
“With X-ray images, we can see the hard minerals, similar to a CT scan with bone fractures. Neutrons give us an image of the fluid that flows in the pores of the rock,” says Shafabakhsh.
A neutron image of an arm would show the blood flowing through the veins, but it would be harmful to humans.
He has examined these rock samples at two laboratories in Grenoble, France, and Zurich, Switzerland. Both are synchrotrons, machines that combine X-rays and neutrons.

Shafabakhsh says that people in the industry often ask why they study the process on such a small scale.
“Traditional models that only look at field scale tend to overestimate the reactions taking place in the field. So to get an accurate picture, we need studies on a small scale,” says Shafabakhsh.
He adds that industry will face significant problems with CO2 leaks if they do not take into account what happens on a small scale.
Researchers in the basalt project are also conducting idealised experiments on how CO2 moves in the pores of basalt.
A laboratory model illustrates the principle
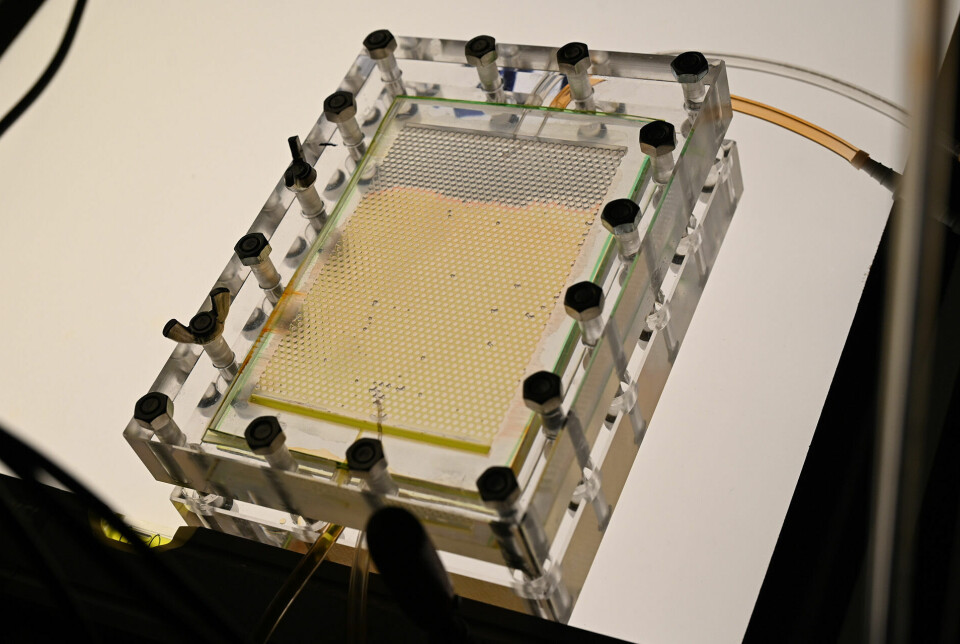
In the basement of the Physics Building, Yao Xu has 3D-printed models of rock into which they can inject CO2 and fluids to study the patterns in which CO2 moves in detail.
“CO2 has its own personality. The gas has things it likes and dislikes. It likes water,” says Xu.
He injects CO2 at the top of his setup. CO2 reacts with water and becomes heavier, causing it to naturally sink downward.
Xu can adjust pore size and the rate at which he injects the CO2 gas. A camera takes a picture every 30 seconds during the six-hour duration of each experiment.

The results from the lab experiments and measurements on rock samples will eventually be compiled and compared with the work being done on the field scale.
From nanoscale to entire reservoirs
Project leader Francois Renard explains that the amount of CO2 that will react with the rock, and thus how much can be stored, depends on how porous the rock is. This depends on the minerals it contains, its permeability, and a variety of other properties.
"We therefore need fundamental knowledge on multiple scales, from the nanoscale to entire reservoirs," he says.
And he is clear that CO2 storage in basalt could become an important part of the climate solution.
References:
Birgisson et al. Mapping dissolved carbon in space and time: An experimental technique for the measurement of pH and total carbon concentration in density driven convection of CO2 dissolved in water, Advances in Water Resources, vol. 198, 2025. DOI: 10.1016/j.advwatres.2025.104916
Johannesen et al. Revisiting the Stratigraphy and Structure of the Faroe Islands Flood Basalts for Large-Scale CO2 Storage in Basalt Reservoirs, Basin Research, vol. 37, 2025. DOI: 10.1111/bre.70032
Rosenqvist et al. Reservoir properties and reactivity of the Faroe Islands Basalt Group: Investigating the potential for CO2 storage in the North Atlantic Igneous Province, International Journal of Greenhouse Gas Control, 2023. DOI: 10.1016/j.ijggc.2023.103838
Rosenqvist et al. The architecture of basalt reservoirs in the North Atlantic Igneous Province with implications for basalt carbon sequestration, GeoScienceWorld, 2024. DOI: 10.1144/SP547-2023-96
———
Read the Norwegian version of this article on forskning.no

This content is paid for and presented by the University of Oslo
This content is created by the University of Oslo's communication staff, who use this platform to communicate science and share results from research with the public. The University of Oslo is one of more than 80 owners of ScienceNorway.no. Read more here.
More content from the University of Oslo:
-
Queer opera singers: “I was too feminine, too ‘gay.’ I heard that on opera stages in both Asia and Europe”
-
Putin’s dream of the perfect family
-
How international standards are transforming the world
-
A researcher has listened to 480 versions of Hitler's favourite music. This is what he found
-
Researcher: "AI weakens our judgement"
-
New, worrying trend among incels, according to researcher




































