This article is produced and financed by NTNU Norwegian University of Science and Technology - read more

Water was a winner in capturing CO2
Reducing the level of CO2 in the atmosphere will probably require carbon capture. A surprising substance just might be the ticket.
Climate worries go hand in hand with CO2 emissions concerns. Emissions hit an all-time high last year. The CO2 level in the atmosphere may be higher than it’s been in 3 million years.
Carbon capture will most likely be necessary to reduce the level of CO2 in the atmosphere. To accomplish that we need the technology and materials to do the job. Recently a promising and surprising new candidate has emerged.
"The results are first and foremost important in terms of climate change," says Professor Liyuan Deng at NTNU’s Department of Chemical Engineering.
Professor Deng is leading the work of the membrane research group at NTNU, and their results are gaining attention.
Water altered the material
Power plants that use fossil fuels require a membrane that can filter the emissions and separate out the carbon. These membranes need to be both permeable for CO2 and also separate the CO2 from the other gases, such as nitrogen.
“We didn’t think this membrane material was going to be suitable,” says Deng.
But a simple move changed that. The hopeless membrane candidate needed another substance to work properly. This second substance was simply – water.
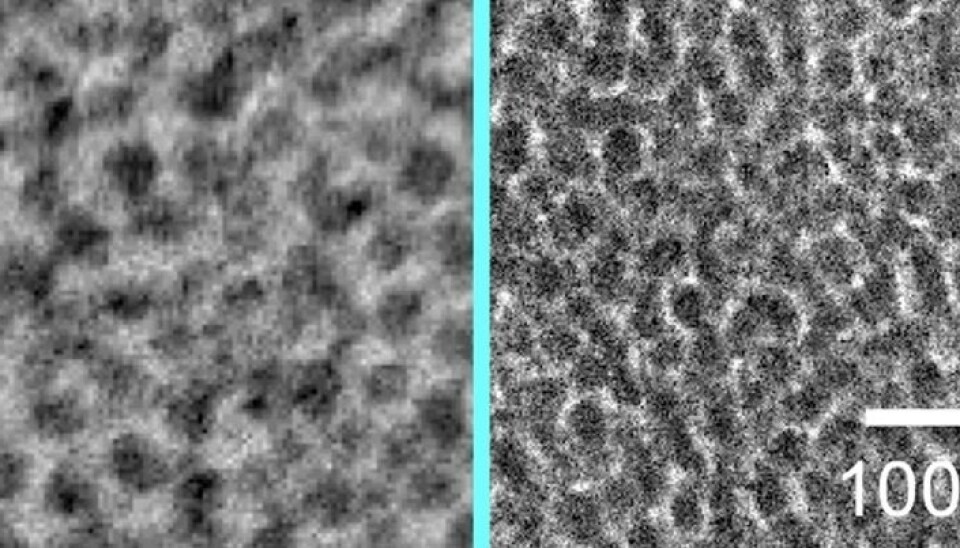
By lowering the membrane into water and then drying it out again, the membrane underwent a change. CO2 penetrated the membrane much more efficiently, and the membrane was somewhat better at filtering out nitrogen.
NPG Asia Materials, a journal in the Nature group, recently published an article on the NTNU research, which stated that “these nanostructured membranes constitute promising candidates for gas separation technologies aimed at CO2 capture."
TESET
The material in question is a polymer. Polymers are relatively inexpensive and easy to make. Many researchers therefore regard them as promising candidates for separating different gases on the large scale that will be needed. The membranes must also be stable and durable.
A polymer is a substance composed of long-chain molecules. Many plastics are polymers, but they are also found in nature as proteins, cellulose and glass, for example.
This particular polymer bears the name poly[tert-butylstyrene-b-(ethylene-alt-propylene)-b-(styrene-r-styrenesulfonate)-b-(ethylene-alt-propylene)-b-tert-butylstyrene].
Fortunately, someone gave it the nickname TESET instead. The material is already in commercial use and is therefore readily available.
“The company holding the patent is interested in this new field of application,” says Deng.

Only membrane research group in Norway
The Membrane Research Laboratory at NTNU hosts the only group in Norway that is researching membranes from polymers that can be used to filter CO2 from the air. Some individual scientists are working with the same materials, while other groups are looking at inorganic membranes. The research on membranes in Norway as a whole is quite advanced, and perhaps even cutting edge, according to the professor.
This particular research is part of Horizon 2020, the EU's Framework Programme for Research and Innovation.
The group is also working on other promising candidates for CO2 filtration. Among these are membranes made of graphene oxide. Graphene is the world's thinnest and strongest material. It consists of one layer of carbon atoms organized in a hexagonal pattern. The material has many exciting properties, and several groups at NTNU are looking at the practical fields of application for it.
All the published results and articles from the membrane research group associated with Horizon 2020 are freely accessible for the public, and the Trondheim laboratory is open to researchers from around the world to carry out experiments, as long as they receive permission and support from the EU ECCSEL project.
Reference:
Dai, Z., Deng, J., Aboukeila, H. et al. Highly CO2-permeable membranes derived from a midblock-sulfonated multiblock polymer after submersion in water. NPG Asia Mater 11, 53 (2019) doi:10.1038/s41427-019-0155-5


































