THIS ARTICLE/PRESS RELEASE IS PAID FOR AND PRESENTED BY NTNU Norwegian University of Science and Technology - read more
New medicine may extend terminally-ill cancer patients’ lives
It has taken 18 years, but Professor Marit Otterlei has now created a completely new type of cancer medicine. No similar medication has progressed this far in development worldwide.
Otterlei’s group at NTNU has been responsible for the basic research and designed the peptide-drug that NTNU spin-off APIM Therapeutics now has taken to Phase II trials.
It has taken 18 years and more than EUR 20 million.
The medicine has now been tested on 20 cancer patients who were terminally ill. They had tried all available treatments, and as a last resort they opted to try a new option that was in the experimental stage.

Cancer stopped growing
The trials took place in Australia, where there are clinics that specialise in testing new medicines.
The results are very promising and have been published in a recognised cancer research journal.
70 per cent of the patients who received the medicine were stable after six weeks. 12 per cent continued the medication and were stable for 18 weeks. One woman took the medication for 17 months, and was stable for over two years.
In other words, the cancer stopped growing.
The aim of the testing in Australia was not primarily to check whether the medicine worked, but rather to determine whether it was toxic to humans.
Results indicate that it certainly wasn’t toxic.
The medicine has previously been shown to both keep cancer at bay and defeat it in laboratory and animal experiments.
Marit Otterlei is behind all this research. She is a professor of molecular medicine at NTNU.
Patients avoid hair loss
Cancer is a result of damage in the cell’s DNA/genetic material, so that the cells divide uncontrollably. Eventually, damaged cells accumulate and form a cancerous tumour.
“Cancer cells are more stressed than other cells. However, they don’t die but continue to grow even when they are damaged. Conventional cancer treatment with chemotherapy puts more stress on the cancer cells so that the cells eventually do die. Chemotherapy affects all cells, including the normal ones, such as in the hair follicles, and thus affects the whole body with many side effects like hair loss,” Otterlei says.
The new cancer medicine that Otterlei has developed is called ATX-101. It only works on stressed cancer cells, and leaves the other healthy cells in the body alone.
One of the differences with this medicine is thus that cancer patients avoid losing their hair.
“ATX-101 can be used as the only treatment. It can stabilise the cancer as shown in the recently published studies, but the medicine can also help chemotherapy work even better so that you don’t have to have so much of it,” Otterlei says.
From discovery to medicine
Simply put, the new cancer drug destroys cancer cells’ ability to handle stress so that they die or stop growing.
In slightly more complicated terms, the new cancer drug is a peptide that contains a special binding sequence, APIM, which is found in many different proteins that bind to a coordinator molecule called PCNA.
More than 500 proteins can potentially bind to this coordinator molecule.
“Half of the proteins contain the binding sequences that we discovered, and yielded the name APIM for the sequence. We found that the APIM sequence is used to bind to PCNA mainly during stress, and it’s important for regulating stress in the cell. By blocking these bonds, the stress regulation will be destroyed,” Otterlei says.
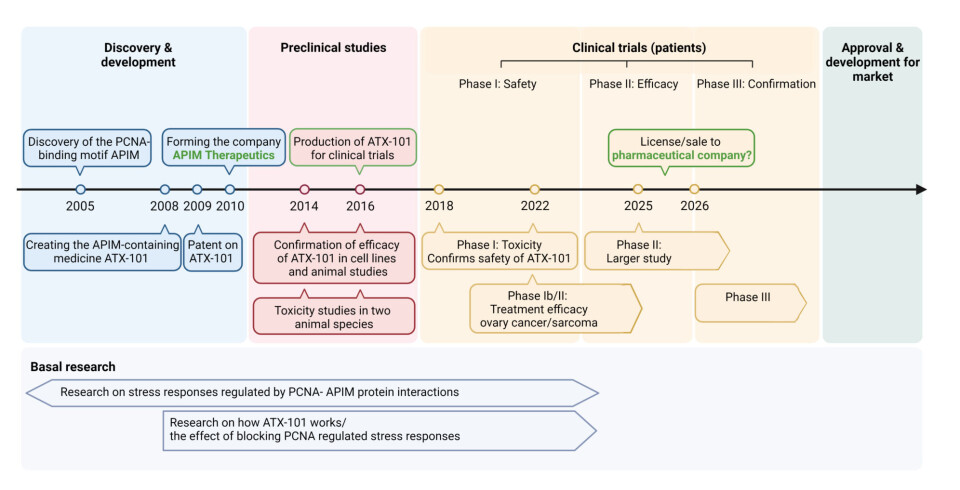
The path from the biological discovery in the lab to applying this knowledge to treat people has taken 18 years.
The first obstacle was to create a medicine that worked in the same way as the biological discovery.
No real pharmaceutical industry in Norway
Otterlei and the team were able to create the medicine after many years of repeated trial and error, which then enabled them to obtain a patent.
The team also worked to understand how the coordinator molecule PCNA regulated different stress functions. Otterlei and her colleagues at NTNU published an article that showed that PCNA has a newly discovered role as a regulator of the metabolism in our cells.
The researchers believe this role is crucial for the stabilising effect that the medicine has on cancer patients.
All the basic research is being carried out at NTNU, but since a university cannot be a commercial actor and produce medicine, Otterlei had to found a company and start a completely different pursuit than academic publishing.
That pursuit was the hunt for money and investors.
“We have no pharmaceutical industry for new medicines in Norway. What we’re working on is too long-term for most investors here. Clinical trials cost anywhere from ten million euros and upwards, and it’s extremely difficult to obtain enough funding. The big pharmaceutical companies want to have results from a Phase 2 clinical trial before they might be interested,” says Otterlei.
It has also been problematic to get support for the basic research surrounding the biological discovery itself, in great part because Otterlei has tried to commercialise and create a medicine.
Effectiveness of the medicine is now being checked
The development has now entered Phase 2. The testing in Australia (Phase 1) needed to show that the medicine was not toxic. Phase 2 needs to establish the effectiveness of the medicine, which is currently ongoing.
The medicine will now be tested in the USA on patients with sarcoma, a type of connective tissue cancer. In Australia, the medicine will be tested on ovarian cancer patients. Both clinical trials are being conducted in combination with chemotherapy.
However, even if the results from the trials are good, the way forward is still uncertain.
“A lot of medicines might work but don’t make it through the development process. The pharmaceutical industry doesn’t take on any projects they can’t profit from. What we develop has to work slightly better than current treatments, and preferably be cheaper to produce and have fewer side effects. Only then can an expensive development run pay off. It’s been a long run, and there’s still a long way to go,” Otterlei says.
References:
Gilljam et al. Identification of a novel, widespread, and functionally important PCNA-binding motif, J Cell Biol., 2009. DOI: 10.1083/jcb.200903138
Lemech et al. ATX-101, a cell-penetrating protein targeting PCNA, can be safely administered as intravenous infusion in patients and shows clinical activity in a Phase 1 study, Oncogene, vol. 42, 2023. DOI: 10.1038/s41388-022-02582-6
Røst et al. PCNA regulates primary metabolism by scaffolding metabolic enzymes, Oncogene, 2023. DOI: 10.1038/s41388-022-02579-1
Read more content from NTNU:
-
This determines whether your income level rises or falls
-
Why is nothing being done about the destruction of nature?“We hand over the data, but then it stops there"
-
Researchers now know more about why quick clay is so unstable
-
Many mothers do not show up for postnatal check-ups
-
This woman's grave from the Viking Age excites archaeologists
-
The EU recommended a new method for making smoked salmon. But what did Norwegians think about this?





































