An article from Norwegian SciTech News at SINTEF
New allergy test promises safer antibiotic use
Allergies to antibiotics are the most common form of medication allergies and, in the worst cases, can result in anaphylaxis and death. Sintef is participating in the development of a new allergy test that will make it easier to provide patients with safe and correct treatments.
As many as ten per cent of the population say that they are allergic to the antibiotic penicillin, although the true figure is probably less than one per cent. Patient over-reporting means that many people who are in fact fully tolerant of penicillin are treated unnecessarily with other, universal, antibiotics which entail a higher risk for the development antibiotic-resistant bacteria.
“A quick and accurate allergy test will make it easy to identify allergy sufferers, and at the same time offer the most precise and effective treatments to those who are in fact tolerant of penicillin and other antibiotic drugs”, says Elizaveta Vereshchagina, a research scientist at Sintef.
Vereshchagina, together with her colleagues Erik Andreassen and Michal Mielnik, is responsible for the Norwegian contribution to the EU project COBIOPHAD, which has been set up with the aim of developing a quick, inexpensive and reliable technique for identifying allergic responses to beta-lactam antibiotics, including penicillin.
Quicker, less expensive, and more reliable
At present, there is no reliable test for the detection of allergies to antibiotics, and the methods used to detect other types of allergies are both expensive and time-consuming. Waiting times for the results of current blood tests for allergies are more than three hours, and the price for each allergen being tested is relatively high.
Sintef is now participating with several European universities, research centres, and commercial companies to develop an allergy test which, if all goes to plan, will be ready to undergo clinical tests in hospitals in five years’ time. The test will enable a blood sample to be analysed in 30 minutes. The aim of the project is to reduce the cost per test for each allergen to ten per cent of current prices.
Measuring allergic intensity
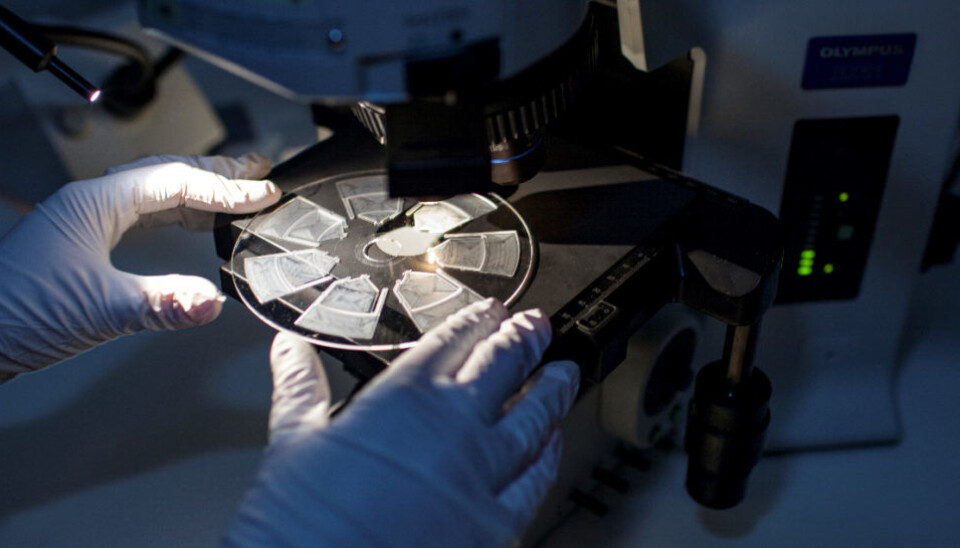
The aim of the project is to develop a measuring device that can identify antibodies in blood samples using advanced photonic technology. The device under development is the size of a small laptop, and works by distributing the patient’s blood sample in small compartments built into a disc-shaped cartridge for analysis. The compartments contain a variety of allergens. If a patient is allergic, the allergens will react with the antibodies in the blood sample.
The reactions between the allergens (pre-loaded into the compartments) and the antibodies in the blood sample are measured by scanning the cartridge with a laser. When a reaction occurs, the device will detect the change and quantify it in the form of a measure of intensity of the patient’s allergic reaction.
The device will be able to test the patient’s blood for many medication allergies at the same time, because it is possible to pre-load several allergens in each compartment in the cartridge. Building several parallel compartments into each cartridge makes it possible to repeat the tests and thus achieve more reliable results.
Designing a microlab in CD-format
At Sintef’s microfluidic laboratory, research scientists are now working on cartridge prototypes. These are made of a plastic polymer and contain between five and ten blood test compartments.
Processes that previously could only be carried out in laboratories can now be performed in the cartridge compartments. The cartridge is designed with microscopic channels (0.1 to 0.8 millimetres wide) within which small volumes of blood move and come into contact with the allergens. It is crucial that the blood reaches the right locations at the right times during the test, and that sample material does not leak from the cartridge. In order to achieve this, it is necessary to have expert knowledge of microfluidics – how fluids behave in constrained channels at very small scales.
“Moreover, we need expertise in polymers and in the manufacture of cartridges with specific requirements in terms of their optical properties”, says Andreassen.
“Our first step is to design a computer model of the cartridge, and then make prototypes using techniques such as machining. In order to test how well the prototype cartridges work, we fill them with fluid, rotate them at high speed, and use cameras to observe how the fluid distributes itself in the channels and compartments”, explains Mielnik.
Fluid flow is governed by the rotating movement of the cartridge. By varying the rotation speed and direction, a blood sample can be moved to different locations on the cartridge.
When the cartridge prototype has been verified, the final version will be manufactured by spray moulding and then made ready for mass production.
“Developing a design that meets the requirements of both allergy tests and the constraints imposed by mass production technologies is proving to be a challenging task”, says Vereshchagina. “But the COBIOPHAD project has set itself ambitious goals. We’re looking to develop a new method of testing allergic reactions to antibiotics in the space of just three years”, she says.
Big opportunities for in vitro diagnostics
When development is complete, this testing technique could be used to test a variety of allergens, and not just those linked to antibiotics. The technology will be assessed during the project by means of clinical tests, and the aim is to achieve commercialisation on completion of the project.
“The technology now being developed as part of this project will generate major opportunities for both the private and public health sectors”, says Vereshchagina. “We are particularly hopeful that Norwegian companies working in the field of in vitro diagnostics will recognise and grasp the opportunities linked to this technology”, she says.
































