This article is produced and financed by NTNU Norwegian University of Science and Technology - read more

Simple nasal spray ready to prevent people from dying of overdoses
Between 250 and 270 people die each year from heroin or opioid overdoses in Norway. In the EU, thousands die. European users now have a better option available for helping each other.
Time is of the essence when a person has overdosed on heroin or other opioids. Mortality is high. But a friend can give an antidote quickly if it’s readily available.
Users and their relatives have been part of the team as Norwegian researchers and industry developed a practical solution for twelve European countries: a nasal spray that not only is easy to store and use, but that has a low risk of withdrawal symptoms afterwards.
"Evidence of effective treatment for everyone, including those who take drugs, has always been our driving motivation," says NTNU professor Ola Dale in the Department of Circulation and Medical Imaging.
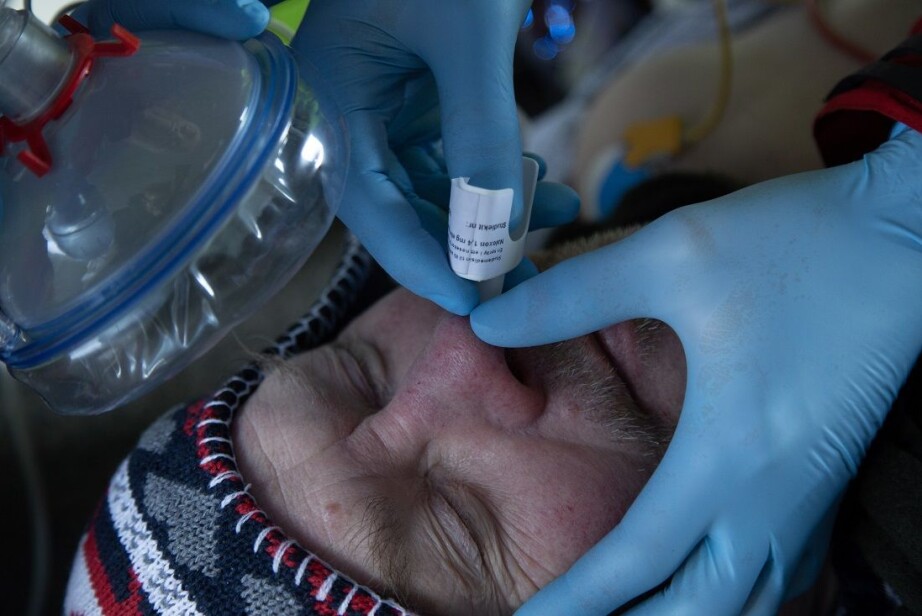
Known remedy, but cumbersome to use
Dale is a specialist in anaesthesiology and clinical pharmacology, and has worked with both painkillers and nasal sprays for a long time as a way to save lives. He wanted to give users a better option to help save each other.
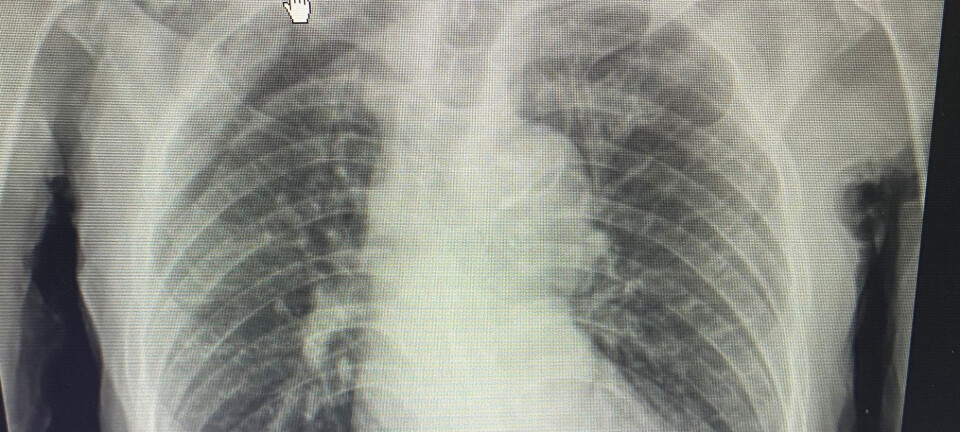
Naloxone is a known remedy for reversing overdoses and preventing overdose deaths. Rescue kits have been used by healthcare workers since the 1970s, and by friends and family since the late 1990s.
But the temporary sprays have been cumbersome to use, and their efficacy has been questionable. Nor did the temporary sprays undergo the normal trials to test their effectiveness and side effects until 2015. Doses that are too high can cause strong withdrawal symptoms later.
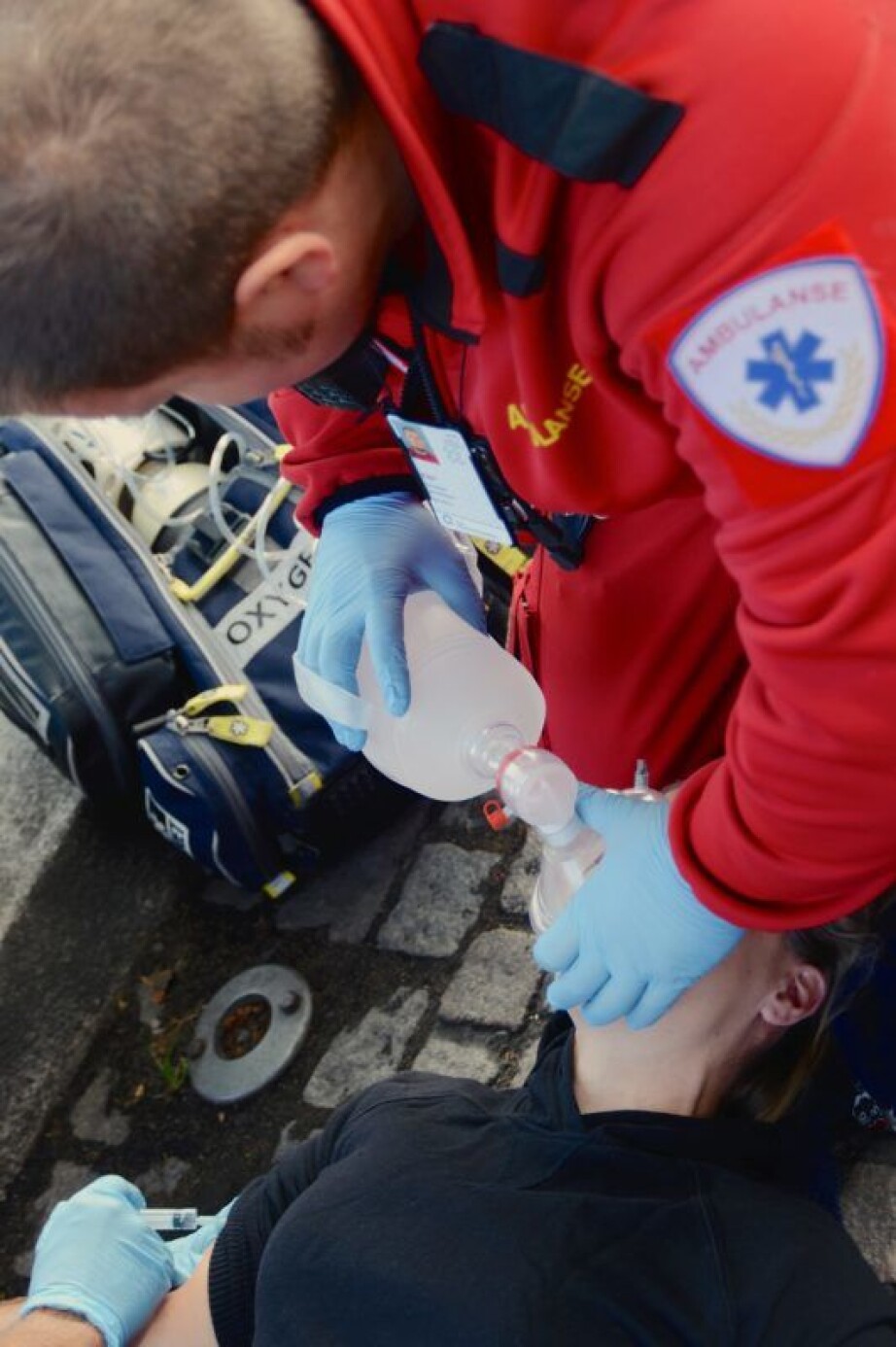
In 2009, Dale became aware of the problem and decided to do something about it. It has taken close to 12 years to reach this point, but since 1 July the nasal spray Ventizolve has finally hit the market.
No dreaded withdrawal symptoms
"Our nasal spray contains a lower amount of naloxone than other approved sprays. We arrived at a dose that is effective in the vast majority of overdose cases, without the overdose victims having to pay the high price of much feared withdrawal symptoms," says Dale.
The work to develop a new nasal spray has met with a lot of resistance during all these years. It was delayed by a flood in Bangkok and had small deviations in the production timeline of the spray. But Dale and his many assistants never gave up.
Dale has been on four research trips abroad, and international contacts have proven useful. Academic pharmacist Phatsawee Jansook in Bangkok took on the task of formulating a spray solution adapted to nasal use.
Later, medical student Ida Tylleskär came on board, and shortly afterwards Arne Skulberg joined as a PhD candidate. He is an anaesthetist at Oslo University Hospital, Ullevål, where the nasal spray could be tested on healthy volunteers.
Skulberg won the national final of the Researcher Grand Prix in 2014 for his work with the nasal spray. Tylleskär defended her doctoral dissertation on nasal sprays and overdoses in August.
To begin with, the research team received help from the Norwegian Institute of Public Health’s "Biopharmaceutical production" vaccine laboratory. The facility closed and in 2014, the researchers realized that they had to find an industry partner.
Launched in Europe
"We would never have succeeded in making our product without an industrial partner coming into the picture. Alone we couldn’t have met the many formal requirements of the Norwegian Medicines Agency," says Dale.
The group therefore contacted Farma Holding/Dne (formerly the Norwegian Ether Factory). Today the company name is called dne pharma.
Dale says that in contrast to the Norwegian Directorate of Health, Farma Holding/Dne believed in the team and the project’s commercial potential.
dne pharma has now ensured that the researchers have secured marketing authorization for Ventizolve in 12 European countries. Drug overdoses are a major problem in Europe.
"In 2017, 8200 people died from overdoses in the EU. If we include Norway and Turkey, we’re talking about 9400 deaths," says Geir Simonsen, CEO of dne pharma.
These deaths often happen to people in their 30s and 40s who die long before their time.
Norwegian production important
"The product is made at Karihaugen in Oslo, and we have no plans to move production out of the country," says Simonsen at dne pharma.
"The importance of safeguarding national pharmaceutical production hasn’t been lost on us recently," says Dale.
Along the way, the product has been thoroughly tested.
Users were advisors
“We wanted to document that the spray is as good as the standard treatment – that is, when the health personnel in the ambulances inject someone who’s overdosed with naloxone,” says Dale.
A study is currently underway in Oslo and Trondheim where the spray is being compared to the standard treatment.
“We couldn’t have carried out this study without dne pharma,” says Arne Skulberg who now leads the study. “The study will include 200 overdose incidents, and we’re close to reaching that number.”
Active opioid users were involved in the planning of the study. The advice they gave made it possible to get the study approved by the Regional Committee for Medical and Research Ethics, says Dale.
“Already at the study design stage, input from the user group helped create a robust study rooted in the user environment,” says Skulberg.
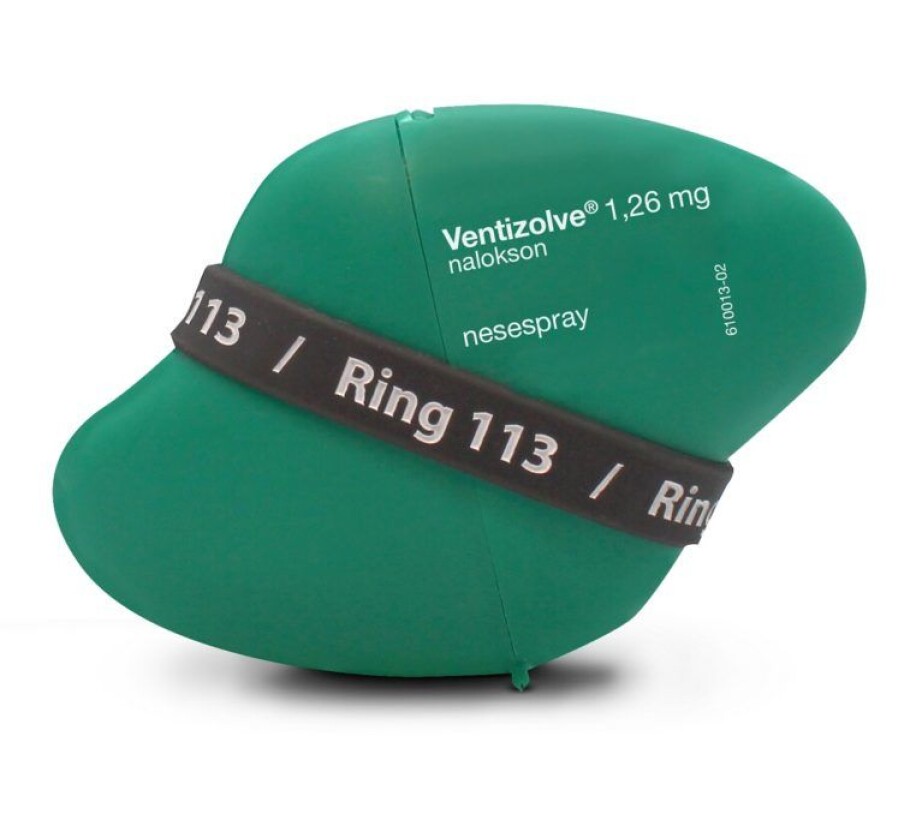
Users also collaborated with the design company Anti in Bergen to design the packaging.
Tom Morgan from the design company Anti created packaging that broke with ordinary pharmaceutical industry thinking. It was designed to function on the users’ terms: easy to carry in your pocket, and easy to recognize on dark and cold days and nights in a bag or purse. A single package also needed to have room to include the spare spray.
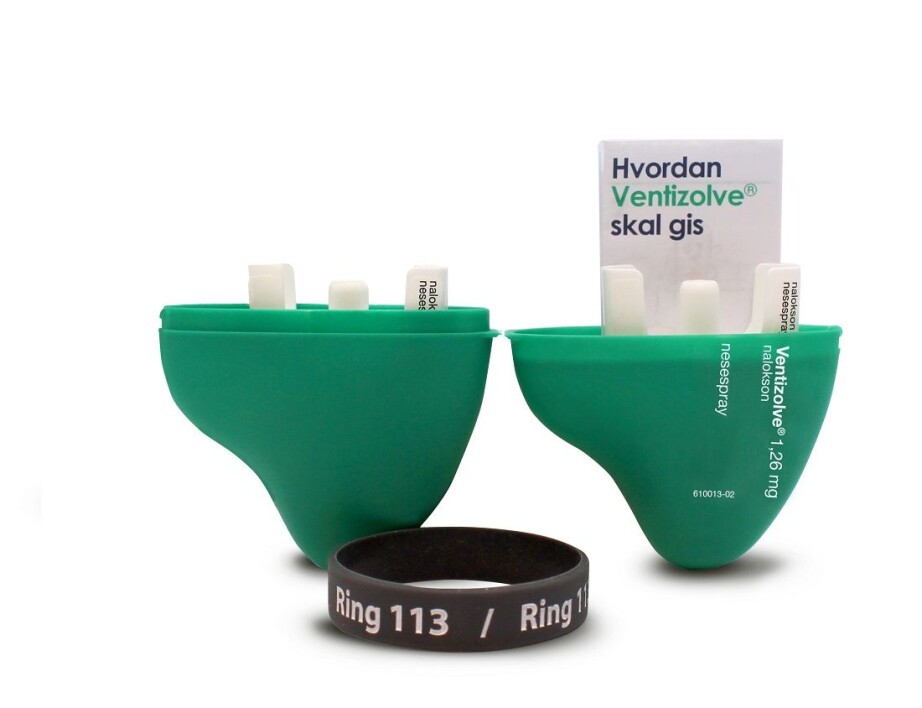
Hope more people will carry spray with them
The hope is that the final product – two sprays containing naloxone and a case – will increase the probability that the spray will become, and continue to be, something that users take with them.
“We’re also hoping for the spray to gain maximum distribution throughout society. The idea is to make the spray extra accessible by giving it to people who are at greatest risk of witnessing an overdose, like users, family members, social workers, security guards and the police,” says Tylleskär.
NTNU on the team
NTNU has been part of the whole research process, starting with the development of an appropriate pharmaceutical formulation, to approval of the drug, and finally to the clinical trials, which are the gold standard for medical research.
“Support from the department heads, first Toril Hernes and later Øystein Risa, at the Department of Circulation and Medical Imaging; NorCrin; and research support at St. Olavs Hospital / NTNU and Oslo University Hospital have been invaluable,” says Dale.
The project has primarily received financial support from Helse Midt-Norge (Central Norway Regional Health Authority), NTNU and St. Olav’s Hospital, as well as the Lærdal Foundation and the Norwegian Air Ambulance Foundation.
Reference:
UiO Duo Knowledge Archive. Pharmacokinetics of a novel, approved, 1.4-mg intranasal naloxone formulation for reversal of opioid overdose—a randomized controlled trial. Skulberg, Arne Kristian; Åsberg, Anders; Zare, Hasse Khiabani; Røstad, Hilde; Tylleskär, Ida; Dale, Ola.