Iron could help make cheaper solar panels
Scientists have developed a new iron compound that could be used in future solar panels, and make them cheaper, lighter, and smaller.
Solar cells convert light into electricity. This can happen in various ways and now scientists have developed another: solar cells based on iron, which is a cheap and commonplace material.
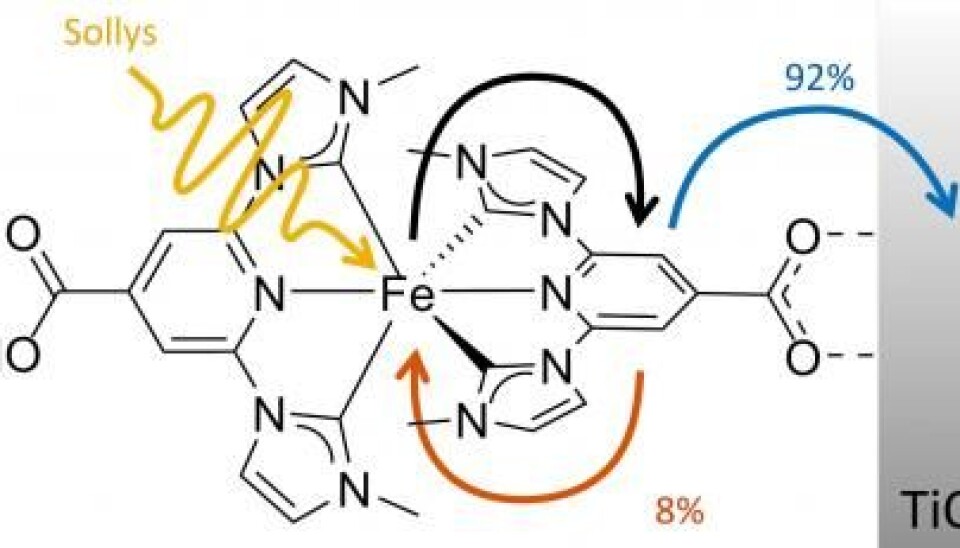
The cells use a newly designed iron compound, which has an iron atom at its centre that effectively spits out an electron when it is hit by light.
The molecule is ideal for use in a specific type of solar cell called Grätzel cells, where the iron replaces ruthenium--an expensive, rare metal.
"The goal of the project was to replace ruthenium with something that’s easier to obtain, which we’ve done," says Tobias Harlang, a postdoc with the chemical institute at Lund University in Sweden.
The new research is published in the scientific journal Nature Chemistry.
Turns the solar cell into a thin film
Common solar cells are made of the semiconductor material silicon. These are quite effective and can convert a quarter of the light energy they receive into electricity. But they are also energy intensive to produce, cumbersome to handle, and there are limits as to where they can be used.
Grätzel cells are somewhat smarter. Instead of using rigid silicon, they are based on colour sensitive molecules that absorb solar energy and transform it into electricity, and they can be placed in a very thin and flexible film.
But, they are not nearly as effective at producing electricity out of sunshine--efficiency drops to 12 to 13 per cent, compared with traditional solar panels.
"If solar panels can be manufactured as a cheap, thin film rather than bulky panels that aren’t even decorative, then they can be fitted to many other types of surfaces--different kinds of building materials, windows, and textiles," says Harlang.
"Grätzel cells also work well at low light intensity and in diffused light, like when it’s cloudy. So they’re great for those of us who live in countries where it’s often overcast," he says.
Iron replaces rare element
The most efficient Grätzel cells are based on molecules built around ruthenium, but this could all be about to change now that a cheaper alternative is available.
"Iron is interesting because it’s just above ruthenium in the Periodic Table, and so it has some of the same chemical properties. Moreover, it’s harmless in the environment and for us, and for each gram of ruthenium, there are 63 tons of iron," says Harlang.
"The problem until now was that molecules based on iron weren’t able to hold onto the energy they produced for long enough. It was simply turned into heat instead of electricity."
Until now, iron-based molecules could only store energy for up to 0.1 picosecond, but the new molecule takes this up to 37 picoseconds. This is enough time to send the energy on as useable electricity.
Colleague: Performance needs to be improved
Associate Professor Torben Lund from Roskilde University, Denmark, notes that the application of these molecules in functioning solar panels is still in its early stages, and their performance as real solar cells is not yet overly impressive.
"There’s a very, very long way to go before we have something resembling a practical application," he says, remarking that the new molecules are only capable of converting 0.13 per cent of the energy in sunlight into electricity.
According to Lund, this figure needs to be at least 50 or 100 times as large before they could have commercial applications.
Harlang will spend the next few years trying to optimise the molecule so that it can extract even more energy out of sunlight. But it will take some time yet before these new solar cells hit the market.
“There is a huge untapped potential in solar energy," says Harlang.
"[Eventually] we’ll be able to fit them to many other places--to the facades [of buildings], or the roofs of cars, even on the back of phones. We will be much better at exploiting solar energy than we are today,” he says.
--------------
Read the Danish version of this article on Videnskab.dk