Synthetic mussel adhesive sticks to anything
A new type of glue that can make any kind of materials stick together is currently being developed by Danish scientists. The glue can even glue wounds together and make objects stick under water – and if it breaks, the glue can repair itself.
A new type of glue, which mimics one of nature’s most versatile forms of sticky mass – the glue that mussels use to stick to just about anything under water – is currently being developed at Aarhus University in Denmark.
The glue in the barbels (whiskerlike feelers extending from the head of the mussel) of the mussel can attach the mussel’s soft muscle tissue to anything from rock to Teflon. It can repair itself if it breaks and it works perfectly well under water, too.
Over the past few years, scientists have succeeded in identifying enough details of the composition of mussel adhesive that it is now possible to copy it – at least in principle.
And now, researchers at the Interdisciplinary Nanoscience Centre (iNANO) at Aarhus Universtiy, headed by Associate Professor Henrik Birkedal, have just received a major research grant to take a closer look at the secret of mussel adhesive – and especially to try and copy the processes involved.
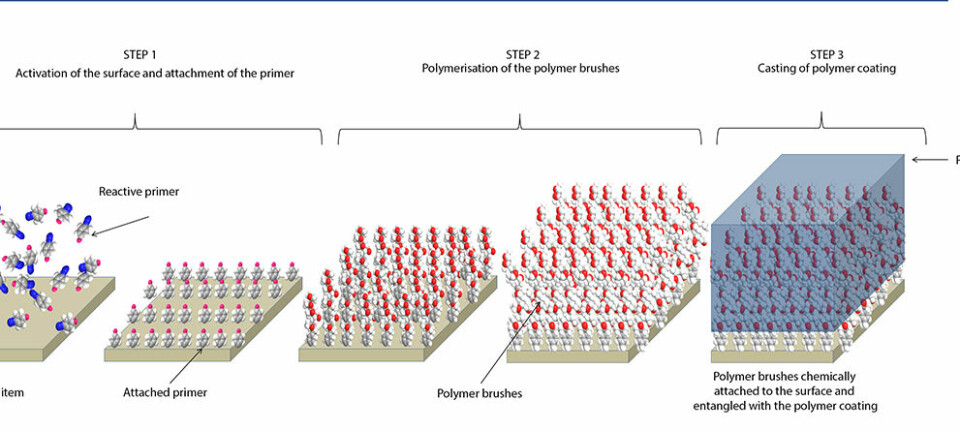
Mussels stick to surfaces by means of a special type of amino acid inside their barbels. We have managed to synthesize this amino acid and we have incorporated it into a polymer, which forms the basis of a glue that has the same unique properties as mussel barbels.
Henrik Birkedal
“Mussels stick to surfaces by means of a special type of amino acid inside their barbels,” says Birkedal.
“We have managed to synthesize this amino acid and we have incorporated it into a polymer, which forms the basis of a glue that has the same unique properties as mussel barbels.”
Self-repairing glue
One of the main ingredients in the new glue is the amino acid known as L-3,4-dihydroxyphenylalanine (DOPA), which accounts for some 20 percent of the proteins in the mussel barbels.
DOPA can bind to a wide variety of materials, but the amino acid binds particularly well to iron, which is an ingredient shared by the mussel barbels and the new synthetic glue.
Our glue addresses a big unsolved problem in terms of glueing tissue together. So it seemed obvious for us to use the glue in the human body. But, strictly speaking, it can be used anywhere you want different types of materials to stick together.
Henrik Birkedal
This means that glue containing DOPA not only binds to other surfaces in various materials – it also binds to itself, and it does so in a very interesting way:
When the glue breaks and the polymers in the glue are torn apart, the bonds between DOPA and iron are cut. However, since DOPA binds strongly to iron, the amino acid re-binds to the iron, binding the polymers back together and thus repairing the broken glue.
Glueing people together
One obvious application for the new self-healing glue would be to glue tissue together after e.g. an operation or to close wounds after accidents.
Here, the challenge consists of making the glue stick to both wet and dry surfaces.
The idea is that we can apply the glue while it’s in a liquid state. This will better enable it to get down into all the microscopic gaps and structures in the surface. We then make the surroundings slightly alkaline, which turns the liquid into an adhesive that now has a strong grip on the surface.
Henrik Birkedal
Not many types of glue can do that, but this is a problem that the mussel solved for the researchers millions of years ago, as DOPA is naturally designed to work under water.
Another medical advantage of the new glue is that the polymer inside it is biodegradable over time, and since the glue can repair itself, it will constantly be able to repair any breaks in the glue until the polymer is completely dissolved and the wound has formed scar tissue.
”Our glue addresses a big unsolved problem in terms of glueing tissue together,” says Birkedal. “So it seemed obvious for us to use the glue in the human body. But, strictly speaking, it can be used anywhere you want different types of materials to stick together.”
Glue changes its shape
A part of the innovative design of the new glue is its ability to change from being liquid like water into a sticky gel.
This process is controlled by some other amino acids that the Danish researchers have also found in the mussel barbels.
These amino acids cause the polymer to become soluble in an acidic solution that has a low pH value. In other words, they cause the polymer to stop functioning as an adhesive.
When the pH value increases and starts approaching neutral or slightly alkaline, the structure of the polymers changes, and the liquid turns in to a sticky and adhesive gel.
This gives the glue an extra tight grip on surfaces.
”The idea is that we can apply the glue while it’s in a liquid state. This will better enable it to get down into all the microscopic gaps and structures in the surface,” says the researcher.
“We then make the surroundings slightly alkaline, which turns the liquid into an adhesive that now has a strong grip on the surface.”
Glue to be tested on animals
The researchers have already ’invented’ the glue, but it is not perfect yet. Over the next three years, they aim to learn how to control the glue so that it functions just the way they want it to.
This involves gaining full control of when it changes from being liquid into being a gel. The researchers will also be optimising the adhesiveness for a variety of types of materials.
To achieve this, they will be testing various types of metal in the glue and also various concentrations of all the components involved.
”We hope that within the next three years we’ll have a formula that can glue tissues together in animals. If we achieve this, then we may well have an adhesive that can glue any types of material together.”
---------------------
Read the Danish version of this article at videnskab.dk