THIS ARTICLE/PRESS RELEASE IS PAID FOR AND PRESENTED BY SINTEF - read more
Developing a new cure for lung cancer with mRNA technology
Lung cancer is one of the most dangerous forms of cancer. Treatments are available, but they are demanding on patients and less than 30 per cent survive. But mRNA technology is offering new hope for higher survival rates because treatments target the malignant cells in an entirely new way.
In Norway, more than 3,300 people contract lung cancer every year. It is the second most common form of cancer among both women and men, after breast and prostate cancer, respectively. It also exhibits the highest cancer mortality rates in both sexes, according to Norway’s foremost medical encyclopedia (Store medisinske leksikon).
“Treatment for lung cancer is often difficult because of tumours that fail to respond to radio- or chemotherapy. Survival prognoses are poor even though patients get the best treatments currently available,” says SINTEF researcher and Project Manager Francis Combes at SINTEF Industry.
A more targeted and effective treatment
The work being carried out by Combes and his colleagues offers new hope. They are working to improve the delivery to cells of so-called therapeutic mRNA as a treatment for lung cancer. This method is a more targeted and effective treatment approach. You have probably heard that mRNA technology has played a key role in the development of the Covid vaccines.
But first of all, a brief refresher about how mRNA works:
The ‘m’ in the name stands for ‘messenger’, while the term ‘RNA’ represents a gene sequence or, more correctly, the body’s temporary copy of a gene sequence. The mRNA molecule contains part of our entire gene system and carries instructions to our cells on how to make certain proteins. In principle, we can use this system to get our bodies to make all the proteins they need. When its messenger task is completed, the mRNA molecule disappears entirely naturally.
The mRNA in the Covid vaccines is used to get the body to make one of the proteins contained in the virus so that we don’t have to inject the virus itself. The idea of using mRNA in a medical context is relatively new, and the technology offers the possibility of treating a number of diseases.
“I will go so far as to say that this represents a breakthrough technology in modern medicine,” says SINTEF researcher Francis Combes. “The mRNA technology makes it quicker and cheaper to manufacture drugs, as all we have to do to make a new protein the body needs is to change the mRNA sequence,” he says.
Tailoring the shape of the molecule
This specialist field is called nanomedicine, and researchers at SINTEF and across the world are currently working to encapsulate mRNA in lipid nanoparticles. Lipids are small fat molecules that act as a form of packaging for the active drug component. They are accepted by our bodies and have the ability to release the active component they contain. This mechanism gives us the opportunity to inject active mRNA molecules into our bodies and deliver them to predefined destinations where they can do their job.
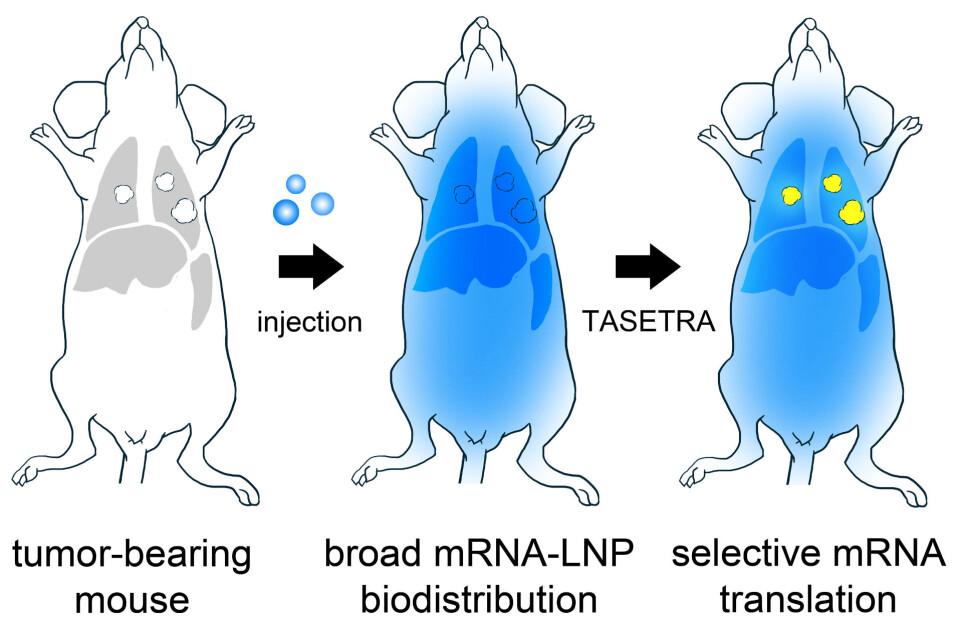
It is precisely this mechanism that the researchers at SINTEF are looking into. Their aim is to deliver the drug safely into the cancer cells where it can work both locally and as effectively as possible. The name of the project is TASETRA, which is an abbreviation for ‘TArgeting by SElective TRAnslation’, and the selective translation aspect is all about tailoring the actual shape of the mRNA molecule.
This can be explained in simple terms by imagining a three-dimensional jigsaw puzzle in which two pieces fit together – the first piece being the cancer cell and the other the drug. When the two become interwoven with each other the drug can work very effectively.
“The therapeutic component of the mRNA sequence has a specific shape such that it is only activated by the cancer cells in the patient’s lung,” Combes explains. “Healthy cells are thus left unaffected, even though they are also invaded by the mRNA. As soon as the mRNA component invades a cancer cell, it is translated into a protein that renders the cancer cell benign,” he says.
The researchers are thus using the physical structure of the molecules to attack the harmful cells.
Hope for multiple new treatments
If the researchers succeed in completing this project, in a few years’ time we may be seeing a drug targeted at a broad range of cancer cells, and possibly also treatments for other forms of disease.
“The TASETRA project is offering us a tool that makes it possible to tailor a drug that can be made effective against specific tissue and cell types by utilising the mRNA code,” says researcher Francis Combes.
But number one on the researchers’ list is to find a cure for lung cancer.
See more content from SINTEF:
-
Old brickwork can be brought back to life in old heritage buildings
-
Researchers surprised by the reactions of penned fish to robots
-
Researchers have run over 1,000 tests on Norwegian windows: This is why rainwater leaks into homes
-
Is it safe to feed farmed salmon with bristle worms raised on fish waste?
-
A solar storm can render your phone useless
-
Researchers want to create a forecasting system for oxygen concentrations in sea water