An article from The Academy of Finland

Fighting hospital bacteria with viruses
Bacteria can quickly pass on their antibiotic resistance on to other bacteria and so create super bacteria. But a promising new method may help solve this problem.
We’re fighting a losing battle against disease-causing germs, with hospitals having to deal with bacteria that are resistant to all known antibiotics.
New antibiotics are unlikely to provide a quick remedy because the development of antibiotics is a slow process.
One would expect that antibiotic resistance is a matter of vital importance to bacteria; so why do they voluntarily hand over a crucial competitive edge to other bacteria?
“In the bacterial cell, natural selection only applies to a certain element,” explains Matti Jalasvuori, a postdoctoral scientist in molecular biology at the University of Jyväskylä, who is working on a new method to hamper the development of super bacteria.
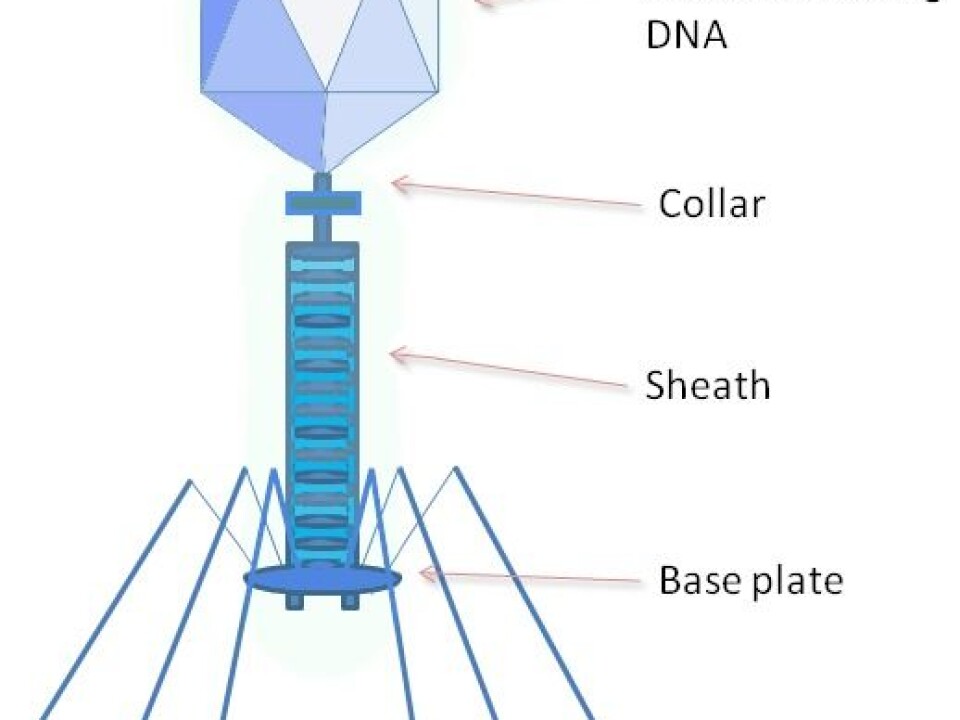
“The parasitic plasmid within the bacteria takes advantage of the cell,” he explains. “Carrying all its genetic information, it moves selfishly from one bacterium to another, with no concern for the fate of its original host bacterium. In the process, it makes the next bacterium resistant to antibiotics as well. In the presence of an antibiotic, this is a favourable quality that can be passed on.”
Targeted natural selection
Some viruses destroy only plasmid-containing bacteria; these are called bacteriophages. Bacteriophages kill bacteria that contain antibiotic-resistant genes, thus improving conditions for other bacteria.
“Bacteriophages cause selection pressure that favours non-plasmid bacteria, which leads to a rapid loss of antibiotic resistance in most bacterial cells,” explains Jalasvuori. “It also seems that the transfer of resistance from one bacterium to another comes to a halt.”
Jalasvuori had a very simple research design: one bacterium and one bacteriophage were grown together to see what happens to antibiotic resistance. He found that the resistance collapsed within just ten days.

A more challenging research design, he says, involves growing a larger number of bacteria to see how quickly the antibiotic resistance transfers from one super bacterium to another bacterium.
“We then add an antibiotic to the cultivation. Next we add a virus to determine whether it can prevent the transfer of resistance. Our unpublished results suggest that it can indeed, and very quickly so.”
Useful for further study
Jalasvuori admits, however, that things are not quite as simple and straightforward as they may seem.
“In the real world, viruses and bacteria live and act together. There are many genetic elements that are constantly transferring from one bacterium to another and that survive even in the presence of bacteriophages.”
Perhaps in the future, we’ll be able to create ecologically adverse conditions for the survival of the element that spreads antibiotic resistance in hospital environments.
Matti Jalasvouri
Jalasvuori believes his experiments show that under certain conditions and in the presence of certain factors, antibiotic resistance does not disappear after all. Even so, his team is getting valuable information about these processes and their causes which will be useful both for our understanding of ecology and evolution and for possible practical applications.
Now working in Australia, Jalasvuori is continuing the theoretical modelling of his research.
“My aim is to deepen our understanding of bacteria and their natural communities,” he says. “My research will shed light on factors that facilitate the transfer of antibiotic resistance and its long-term persistence. Perhaps in the future, we’ll be able to create ecologically adverse conditions for the survival of the element that spreads antibiotic resistance in hospital environments.”
Through a controlled use of antibiotics and by tackling antibiotic resistance, Jalasvuori believes scientists should be able to create a situation where we would no longer need to fear antibiotic resistance as much as we do today.